Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Katelyn Billings
Synthesis strategist is helping hunt for new drugs with DNA-encoded libraries
by Bethany Halford
August 25, 2019
| A version of this story appeared in
Volume 97, Issue 33


Vitals
Current affiliation: GlaxoSmithKline
Age: 30
PhD alma mater: Yale University
If I weren’t a chemist, I would be: “A physical therapist. After going through many hours of physical therapy thanks to my softball career, I had considered going into the field to help others recover from their injuries.”
I’ve overcome adversity in the lab by: “When I made my first DNA-encoded library at the Broad Institute, no one there had experience with the DNA-recorded variant of the technology. I spent many hours diving deep into supporting information of various papers to learn the strategies and technical components associated with running reactions on DNA, making a library, setting up selections, and analyzing the results. Ultimately, I was able to set up the necessary capabilities and made and screened my own 600K-membered library.”
Must-have in the lab: “LC/MS: It’s the only method we have at our disposal to analyze (in a high-throughput manner) whether our reactions on DNA were successful. We would be flying blind without it.”
Must-have on the road: “A bag of Life Savers Gummies to eat on the plane.”
3 key papers
“Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis” (J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.9b00669)
“Small-Molecule Studies Identify CDK8 as a Regulator of IL-10 in Myeloid Cells” (Nat. Chem. Biol. 2017, DOI: 10.1038/nchembio.2458)
“Synthesis and Evaluation of Antimigratory and Antiproliferative Activities of Lipid-Linked [13]-Macro-Dilactones” (Bioorg. Med. Chem. Lett. 2010, DOI: 10.1016/j.bmcl.2010.07.083)
There was a time when Katelyn Billings considered herself extremely productive if she set up 10 reactions over the course of a day. Now, she says, “Running a few thousand reactions in a day is no longer out of the ordinary.”
Billings achieved this leap in productivity in her role as a chemist leading the design and construction of DNA-encoded libraries for pharmaceutical giant GlaxoSmithKline. DNA-encoded libraries are collections of millions of small molecules, each attached to a short strand of DNA that encodes the instructions for making the small molecule. Those millions of small-molecule-DNA conjugates are tested all at once against a single disease-related protein. Scientists then use the DNA to decipher which compounds bind to the protein target.
DNA-encoded libraries have been around for more than 25 years, but they’re enjoying a resurgence in popularity. That comes in part as drugmakers look to overcome drug targets that have eluded conventional high-throughput screening methods. And Billings and her colleagues have set their sights on creating better, more diverse libraries.
In the 3 years she’s been at GSK, Billings has been involved in the creation of 10 libraries, ranging from 1 million to 225 million compounds. On any given day, she might strategize which reactions or building blocks to use to construct a library, decode the results of a recent screen, or create a new chemical reaction for library building.
“What drew me to DNA-encoded libraries was the ability to impact many different projects,” Billings says. The libraries she makes become the starting point for finding small-molecule drugs for the many diseases, such as cancer and HIV, that GSK wants to tackle.
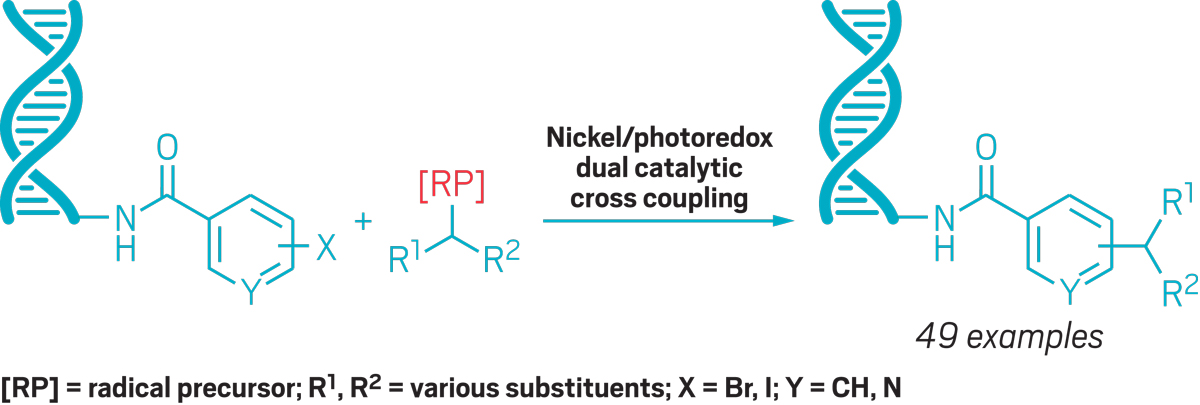
To expand libraries’ diversity, part of Billings’s job is to translate traditional and cutting-edge organic reactions into transformations that can be done with DNA present. This kind of chemistry requires an organic chemist’s nemesis: water. To that end, Billings recently collaborated with the University of Pennsylvania’s Gary Molander on a project that uses photoredox chemistry to couple sp3-centered radicals to DNA. Billings thinks this approach will be useful for a wide range of building blocks.
“This is really a phenomenal technology,” Molander notes, and Billings, he says, “is probably one of the best people in the world at doing it.”
Billings is also working on a computational approach to help construct better DNA-encoded libraries. She says the goal is to strike a balance between selecting every possible building block and selecting only the small subset of those guaranteed to have good properties.
“She is a superb scientist, and I would point to her flexibility and willingness to take on things that she’s not expert in and really bear down on the toughest of problems with a smile on her face,” says Andrew Phillips, who is CEO of C4 Therapeutics and was Billings’s PhD mentor at Yale University.
Billings credits much of her success to her ability to work with a team. It’s a skill she developed as the pitcher of her high school’s softball team, which won the Vermont state championship her junior and senior years.
“No one person could drive the team forward to success. You really all had to work together and rely on each other,” Billings says. “I think that team-sport mentality translates well to work in industry, where we all have to work together.”
There was a time when Katelyn Billings considered herself extremely productive if she set up 10 reactions over the course of a day. Now, she says, “Running a few thousand reactions in a day is no longer out of the ordinary.”
Billings achieved this leap in productivity in her role as a chemist leading the design and construction of DNA-encoded libraries for pharmaceutical giant GlaxoSmithKline. DNA-encoded libraries are collections of millions of small molecules, each attached to a short strand of DNA that encodes the instructions for making the small molecule. Those millions of small-molecule-DNA conjugates are tested all at once against a single disease-related protein. Scientists then use the DNA to decipher which compounds bind to the protein target.
Advertisement
DNA-encoded libraries have been around for more than 25 years, but they’re enjoying a resurgence in popularity. That comes in part as drugmakers look to overcome drug targets that have eluded conventional high-throughput screening methods. And Billings and her colleagues have set their sights on creating better, more diverse libraries.
In the 3 years she’s been at GSK, Billings has been involved in the creation of 10 libraries, ranging from 1 million to 225 million compounds. On any given day, she might strategize which reactions or building blocks to use to construct a library, decode the results of a recent screen, or create a new chemical reaction for library building.
“What drew me to DNA-encoded libraries was the ability to impact many different projects,” Billings says. The libraries she makes become the starting point for finding small-molecule drugs for the many diseases, such as cancer and HIV, that GSK wants to tackle.
To expand libraries’ diversity, part of Billings’s job is to translate traditional and cutting-edge organic reactions into transformations that can be done with DNA present. This kind of chemistry requires an organic chemist’s nemesis: water. To that end, Billings recently collaborated with the University of Pennsylvania’s Gary Molander on a project that uses photoredox chemistry to couple sp3-centered radicals to DNA. Billings thinks this approach will be useful for a wide range of building blocks.
“This is really a phenomenal technology,” Molander notes, and Billings, he says, “is probably one of the best people in the world at doing it.”
Billings is also working on a computational approach to help construct better DNA-encoded libraries. She says the goal is to strike a balance between selecting every possible building block and selecting only the small subset of those guaranteed to have good properties.
“She is a superb scientist, and I would point to her flexibility and willingness to take on things that she’s not expert in and really bear down on the toughest of problems with a smile on her face,” says Andrew Phillips, who is CEO of C4 Therapeutics and was Billings’s PhD mentor at Yale University.
Billings credits much of her success to her ability to work with a team. It’s a skill she developed as the pitcher of her high school’s softball team, which won the Vermont state championship her junior and senior years.
“No one person could drive the team forward to success. You really all had to work together and rely on each other,” Billings says. “I think that team-sport mentality translates well to work in industry, where we all have to work together.”
Vitals
Current affiliation: GlaxoSmithKline
Age: 30
PhD alma mater: Yale University
If I weren’t a chemist, I would be: “A physical therapist. After going through many hours of physical therapy thanks to my softball career, I had considered going into the field to help others recover from their injuries.”
I’ve overcome adversity in the lab by: “When I made my first DNA-encoded library at the Broad Institute, no one there had experience with the DNA-recorded variant of the technology. I spent many hours diving deep into supporting information of various papers to learn the strategies and technical components associated with running reactions on DNA, making a library, setting up selections, and analyzing the results. Ultimately, I was able to set up the necessary capabilities and made and screened my own 600K-membered library.”
Must-have in the lab: “LC/MS: It’s the only method we have at our disposal to analyze (in a high-throughput manner) whether our reactions on DNA were successful. We would be flying blind without it.”
Must-have on the road: “A bag of Life Savers Gummies to eat on the plane.”
Research at a glance
Credit: Yang H. Ku/C&EN
To build new DNA-encoded libraries, Billings develops reactions like this one to create novel compounds linked to DNA tags without damaging the nucleic acid.
Three key papers
“Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis”
(J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.9b00669)



















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter