Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Covid-19
Podcast: How the coronavirus could disrupt the drug supply
Hear what C&EN’s pharmaceutical editors have learned about how the coronavirus is affecting drug production in China and across the globe
by Lisa M. Jarvis & Rick Mullin
March 10, 2020

As the novel coronavirus responsible for causing COVID-19 continues to spread, questions about the virus, the disease, and its impacts on our daily lives mount. To help you stay current with the science, policy, and business implications of this outbreak, C&EN has made all of its coronavirus coverage freely available at cenm.ag/coronavirus. And in the latest bonus episode of Stereo Chemistry, we discuss one of the largest questions on the business front: How is the coronavirus affecting the global drug supply? Rick Mullin and Lisa M. Jarvis bring you the latest.
Subscribe to Stereo Chemistry now on Apple Podcasts, Google Play, or Spotify.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe
Matt Davenport: Hi. This is Matt Davenport, a producer of Stereo Chemistry. Before we get into this bonus episode about the coronavirus and the pharmaceutical supply chain, I want to point out that we recorded it and published it in early March. The situation is ever changing, and we encourage you to visit and bookmark cenm.ag/coronavirus for the most up-to-date news. Thank you.
The following is the script for the podcast. We have edited the interviews within for length and clarity.
Nancy Messonnier: We expect we will see community spread in this country. It’s not so much a question of if this will happen anymore, but rather more a question of exactly when this will happen and how many people in this country will have severe illness. I understand this whole situation may seem overwhelming and that disruption to everyday life may be severe.
Lisa Jarvis: That was CDC official Nancy Messonnier, and she was addressing reporters about a topic that for weeks has dominated headlines: Coronavirus.
I’m C&EN pharmaceuticals editor Lisa Jarvis and you’re listening to Stereo Chemistry. In this bonus episode, we’re going to dig into a question that the novel coronavirus outbreak has put front and center: Should we be worried about the security of the world’s drug supply?
To answer this, I’ll be joined by senior editor Rick Mullin, who has spent years covering the business of manufacturing our medicines. Over the last month, Rick has talked to a lot of folks about how coronavirus could affect the pharmaceutical supply chain.
But before we get into that, a little backdrop. The world first started hearing about this novel virus in January, a few weeks after a cluster of mysterious illnesses emerged in Wuhan, China. As of March 5, more than 95,000 people had been infected, and nearly 3,300 had died. While the majority of those cases are in China, and in particular in the Hubei province where Wuhan is located, we’re seeing a rapid escalation around the world—particularly in places like Italy, Iran, Japan, and South Korea.
Countries are taking dramatic steps to try to rein in the spread. And that started with China, where entire cities were virtually shut down for weeks. The thing is, we rely on China for a lot of stuff, including the medicines we take. US and European pharma companies might be the ones primarily inventing and marketing new drugs, but along the way, they enlist many partners—you might hear Rick and I call them contract manufacturers. And those folks are responsible for some or all of the steps involved in making an active pharmaceutical ingredient, or API. That’s the actual medicine inside your pill. Historically, those partners had been in Europe and the US. But today? Big chunks of that work has shifted to India and China. Which brings us to coronavirus.
Rick, walk me through why these massive shutdowns in China due to coronavirus have people pretty worried about the drug supply in the United States.
Rick Mullin: I think the best thing to do would be to just start with a little bit of the background and the history and, you know, who the players are in the chemical industry. You know, when you get a pill from your pharmacy that says drug company X on it, it is very likely that the pill was not manufactured by drug company X. Most likely it’s the end result of a supply chain of manufacturing that includes companies all over the world, each supplying chemicals used at different stages of manufacturing the drug. This results from a trend of some 25 years of major drug companies exiting manufacturing, which they’ve done pretty thoroughly at this point.
Active pharmaceutical ingredients, or APIs, are purchased for the most part from contract manufacturers, an interesting sort of corner of the chemical industry that began many years ago as sort of a garage industry in Europe in Italy and in Spain and grew to some major companies in Germany, Switzerland, and also moved to Asia. And a lot of drug chemicals are purchased now from China, which for many years offered understandably the low cost option. But in recent years a couple of things have changed the playing field.
One is that Western producers have really come to the realization that you have a quality issue for the price that you pay or for the savings that you accrue in purchasing from Asia. Another thing that’s happened, interestingly, is that most of the US companies have been purchased in a round of acquisitions mostly by European companies. So interestingly it’s a Europe and China picture to start with. And there has been a lot of concern about China not just having to do with the recent coronavirus. A year and a half ago there was an environmental crackdown by the government that closed a lot of facilities.
Lisa: I wondered if we could talk a little bit about the different steps in making a drug. There’s, I think, the raw materials, then the intermediates. Eventually you get to the more complicated chemistry there, which is the active pharmaceutical ingredient. Maybe you could give us a sense of which part in that process we tend to rely most on China.
Rick: That’s hard to say. As you mentioned, there is a lot that goes into making the pill. Counting from the top, you’ve got formulation, you’ve got active pharmaceutical ingredients, you’ve got raw materials, you’ve got precursors to those raw materials. All those things are done in China. China tends to be most involved at the lower part of the chain but is involved really throughout.
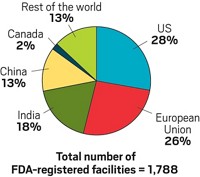
Lisa: And so I think we had a little factoid here about the percentage, though, of our drug supply that we know, at least based on what FDA tells us and what some of your sources tell us, about how much comes from China. Maybe you could walk us through that.
Rick: According to a recent report by the European Fine Chemicals Group, which is an association representing fine chemical makers and drug chemical makers, over 80% of the chemicals that are in pharmaceuticals came through China, were manufactured in China. Even if the supplier is Indian, it’s very likely that precursor materials, starter materials came from China.
Lisa: So, you know, it seems pretty clear that Chinese suppliers represent a large swath of the drug supply chain. So you’ve been reporting a lot on how coronavirus is impacting that supply chain. Could you talk a little bit to us about what you’re hearing from either the customers, whether it’s a contract manufacturer that’s getting raw materials or intermediates from China, or pharma companies. You know, give me your sense of what you’re hearing is happening.
Rick: Let me go around on a couple of recent calls that I’ve made to contract manufacturers of active pharmaceutical ingredients that are in interesting places given where the virus has moved from China. There is an Italian company called Flamma. It’s a family-owned company, typical of the types of companies that really got this industry started. They also have a relatively new manufacturing plant in Dalian, China which is about 1,400 km from Wuhan. And on Feb. 1, the company told customers that Chinese authorities had the plants shut down past the normal New Year holiday closure, which was on Feb. 8. And the company also warned that isolation measures and restrictions on air travel and transportation will likely impact supply logistics beyond the extended holiday shutdown. However, they do have the plant in Italy, and they were serving customers, they had inventory. They issued a statement late last month after cases of coronavirus infection were identified in Venice focused more on their headquarters near Milan.
And like every other company I talked to, they said, you know, we’ve got our plants in operation. We have inventory. We’re watching it very closely. But among the measures that they’re taking now in Italy is limiting visits from customers and others to plants, doing meetings through voice or video conferencing, tightening restrictions on vehicles entering the plant. Also in Italy, closer to Venice, is a company called FIS which is in Vicenza, which is 75 km from Venice. It’s also in full operation. And Giuliano Perfetti, who’s the director of marketing and business development there, says that only one worker was obliged by local authorities to stay home. And the company is taking measures similar to Flamma in regards to face-to-face meetings.
And I also spoke with Guy Villax who’s the CEO of a Lisbon-based company called Hovione, which also has a plant in Macau. And he reports that shipments from Guangdong province seem to have been arriving, but they’re concerned about other provinces over the next few weeks from which they expect to receive shipments. Again, the plants are operating; the plant in Lisbon is fine, Macau is operating. But Villax’s concern, shared by everyone, is not the operation of the plants. It’s transportation.
He notes that once the driver leaves the province and tries to come back in, he goes into quarantine. There is real uncertainty about shipments in the weeks ahead. And he also pointed out something very interesting, reflecting the supply chain, and that is one might now go to a supplier in Germany and find that that supplier is not accepting purchase orders. Why? Because they can’t get their precursor materials from China. So supply from China remains a concern, but supply from Europe itself is becoming a real big concern in terms of raw materials.
I think that the industry is well underway in coming up with a collective procedure going forward. Giuliano Perfetti from FIS, who I mentioned earlier, heads a group at the European Fine Chemicals Group that has been looking at China for a year and a half now, going back to the volatility in supply that resulted from the environmental crackdown. Many, many plants over the last 2 years have been closed in China. So the coronavirus obviously turns the heat up.
The group is putting forward several proposed measures, and one is to sort of fast track the approval of alternate supply. So getting a new supplier is a sort of time consuming process because of inspections and certifications that have to be obtained. And also they’re encouraging getting together 5- to 10-year plans to come up with a new supply chain for pharmaceuticals, essentially. To plan to encourage research and development is critical to raw materials or technologies that are produced in Europe, with the aim to reduce the European industry’s dependence on what they term as overseas supplies.
Advertisement
Lisa: One thing I thought it would be worth pointing out is that this isn’t the first time that there’s been a major potential disruption to the pharmaceutical supply chain. For example, back in 2017, Hurricane Maria ravaged the island of Puerto Rico. The widespread destruction created a humanitarian disaster, the consequences of which continue to this day. But there was a side consequence: Puerto Rico is home to many, many pharmaceutical manufacturing sites where companies make some biologics, and do a ton of what’s called “fill and finish” work. That’s what people call it when essentially the active ingredient is sent someplace and then they turn it into the pill form or the injectable drug that you take. And so with widespread power outages, workers unable to get to where they needed to be, and issues getting things in and out of ports, drug companies really scrambled to address the problem. And, I think, were very thrown off-guard. So I just want to point out that while the coronavirus situation is underscoring the vulnerability of our supply chain and our reliance on China, it’s not the only place that we rely on. And it’s not the only type of situation that we should be worried about.
Rick: Right. It is a global supply chain indeed. And what happens in one place affects what happens in another. But when you have such a huge amount of activity coming to one region—China—it certainly is going to, is expected to send a shock through the system. And we’re already seeing that.
Lisa: So that pill that you mentioned at the beginning, is it at risk? Do you think people need to be worried about the drugs that they need to take every day?
Rick: I think it would be fair to say that they don’t have to start worrying about the supply of the drug that they take every day. But this is a situation that’s moving rapidly. Many of the sources I’ve spoken to have emphasized that there’s a huge danger in sort of fear mongering. And I think to say right now that we’ve got a problem with whether we can expect our pills to be here next month is a bit much.
Lisa: The thing I keep wondering about with China is that it feels like we have a cushion that people aren’t worried about at this moment, but I’m wondering when things finally get back to normal, how long it takes us to unwind from that kind of backlog of work that needs to be done. And, you know, how long we’ll feel a little bit the impacts in the supply chain to this. I don’t know if that’s an answerable question.
Rick: Right, that is yet to be seen. You know, there are a couple of consultants that I speak to. I regularly speak to. James Bruno, who is the president of Chemical and Pharmaceutical Solutions, who is very well versed in this. He specializes in the API supply to the drug industry. He told me the good news is that most of the people dealing with China tend to have inventory. But if this doesn’t straighten out in the next 3 months, we can have some real problems with supply disruption.
Another consultant I spoke with earlier this month, Steven Lynn, who is a former head of the FDA’s quality compliance office, observed that API suppliers should actually take advantage of short-term disruptions to review their supply chains and logistic vulnerabilities. And he went so far as to quote Winston Churchill, who said, “Never let a good crisis go to waste.”
Lisa: On that note, thank you so much, Rick, for giving us your perspective from your many years covering the API industry. It’s really helpful. I should point out that this story continues to evolve quickly. In early March, India—the other major drug manufacturing hub we mentioned—said it was limiting exports of certain medicines, most of them antibiotics. FDA, meanwhile, said it had its first report of a coronavirus-related manufacturing shortage. Pharma companies? Well, right now they are saying that things are okay.
So keep an eye out for C&EN’s ongoing coverage of this topic, and so much more. All of which you can find on our website. You can check out all of our reporting on the science, policy, and business angles of this outbreak at cenm.ag/coronavirus. Every story on that page will be freely available for the duration of the outbreak.
And please give us your feedback on this episode and others. I’m @lisamjarvis on Twitter and Rick is @rick_mullin3. You can also email Stereo Chemistry at cen_multimedia@acs.org.
Thanks so much for listening.
UPDATE
This story was updated on March 18, 2020 to ensure listeners are aware of the podcast's original publication date.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter