Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Delivery
Tortoise-inspired, ingestible device flips itself upright to inject insulin inside the stomach
Device could replace ineffective insulin pills and avoid the need for shots, study in pigs suggests
by Tien Nguyen
February 7, 2019
| A version of this story appeared in
Volume 97, Issue 6

As a drug, insulin is hard to swallow. Well, not literally. Oral insulin medications exist, and people take them. But insulin is a hefty hormone. It’s too big to penetrate deep into gastrointestinal tissue and therefore absorb into the bloodstream efficiently, and it succumbs easily to the stomach’s harsh acids and enzymes, breaking down prematurely. People with diabetes often take suboptimal insulin pills for years before their doctors switch them to more effective, but burdensome, insulin injections.
Now, scientists have created a single-use, ingestible device that delivers insulin by injecting it into the stomach lining; the device then passes harmlessly out of the body. They demonstrated that it works in pigs, administering a dose of the drug comparable to subcutaneous injections (Science 2019, DOI: 10.1126/science.aau2277). The research was carried out by scientists at the Massachusetts Institute of Technology, Harvard Medical School, KTH Royal Institute of Technology, and Novo Nordisk.

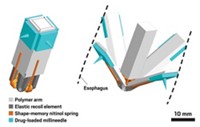
Led by MIT’s Giovanni Traverso and Robert Langer, the team says three unique design features make the ingestible device, called a SOMA (self-orienting millimeter-scale applicator) work: its bulbous shape, drug-release trigger, and special insulin spike.
The device was inspired by the shell of the leopard tortoise, which keeps the animal upright. The SOMA’s curved upper shell widens to a base with lipped corners so that when the device is swallowed, it tumbles to an upright position with the spike pointed downward toward the surface of the stomach wall. Via pig endoscopy, the researchers observed that compared with other shells shaped like spheres or ellipses, the device could consistently right itself.
Traverso says they targeted the stomach because it’s the safest place in the digestive system to deliver the drug. The device reaches the stomach almost immediately. Targeting other parts of the digestive system would be challenging because digestion times vary from person to person, which means they would have less control over when the drug releases, he explains. Also, the stomach’s lining is thick enough that the insulin spike can plunge into its tissue without tearing a hole through the organ’s wall.
Inside the device is a compressed stainless-steel spring attached to an insulin-loaded spike. The spring is held in a compressed state by a sugar-based trigger. The spike is made of a biodegradable shaft of poly(ethylene oxide) (PEO) and hydroxypropyl methylcellulose, capped with a solid tip composed of 80% insulin and 20% PEO packed together under extreme pressure.
Within minutes of the device flipping itself into position in the stomach, the digestive organ’s humid environment dissolves the sugar-based trigger, releasing the spring and jabbing the insulin spike into the tissue. The team found that the device resulted in insulin uptake comparable with manually injected insulin in pigs that had empty stomachs. They saw no signs of tissue damage in the animals.
The researchers say the device is a proof of concept that could be used to deliver any number of valuable biomacromolecule therapeutics, such as RNA- or DNA-based drugs. They plan to keep experimenting with different components of the device, potentially incorporating more biodegradable materials, and hope to test their device in humans within a few years.
Nanoengineer Joseph Wang at the University of California San Diego calls the system remarkable and commends the team’s creative engineering to dramatically enhance oral insulin delivery. “Such ability to deliver insulin orally offers an attractive alternative to subcutaneous injections of insulin,” he says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter