Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
Carbon monoxide can be deadly. But researchers want to use it for good
CO shows remarkable healing activity against an array of illnesses, but scientists need to find better ways to deliver it
by Alla Katsnelson, special to C&EN
November 17, 2019
| A version of this story appeared in
Volume 97, Issue 45

Carbon monoxide is a killer. The colorless, odorless, tasteless gas produced by the incomplete combustion of gasoline or other fuels sends 50,000 people in the US to the hospital each year—about 400 die—when their furnaces malfunction or engines run in improperly ventilated spaces. The Greeks and Romans purportedly used it for executions, and in the 19th century, famed French physiologist Claude Bernard correctly guessed that it exerts its noxious effect by taking oxygen’s place in binding to hemoglobin. That hemoglobin–CO bond is reversible but ferociously strong—210 times as strong as the one hemoglobin normally forms with oxygen in our blood. So when CO is inhaled in large quantities, it blocks oxygen’s access and essentially causes asphyxiation.
At low concentrations, though, CO leads a totally different and more hidden life as a signaling molecule in all living cells. About 10 mL of the gas is naturally produced throughout the human body each day as an iron-containing molecule called heme is metabolized by the heme oxygenase enzyme. In cells, CO interacts with multiple heme-containing proteins that function as cellular sensors and transducers. Through these pathways, CO promotes a surprisingly wide array of beneficial effects. In the past 3 decades, studies in cells and animal models have found that CO can quell inflammation, defend tissue from oxidative stress, prevent cell death, and more.
The list of conditions this tiny molecule might alleviate is so broad that it almost defies belief. The handful of researchers studying CO have reported evidence that it might help treat sepsis, sickle cell disease, complications from organ transplantation, lung fibrosis, ulcerative colitis, cancer, and heart disease, to name a few. “There is no molecule that’s been shown to be this cytoprotective in just about every organ tissue injury—brain, lung, pancreas, heart, kidney, you name it,” says Augustine M. K. Choi, a lung disease expert at Weill Cornell Medical College.
Yet, corralling CO’s potentially curative properties to create viable therapies has proved to be a major challenge. One roadblock has been figuring out the best way to deliver it to the body. Early on, researchers relied largely on inhalation, and multiple clinical trials have shown that inhaling small, controlled amounts of CO is safe. But inhalation would work only in hospital-based situations where clinicians could ensure safety and proper dosage, such as during a transplant surgery or as part of sepsis treatment, and not for chronic conditions.
Now, prospects for CO-based therapies face a make-or-break moment. A handful of efforts are in the works to develop novel molecular packaging to deliver the gas, and two start-up companies plan to launch a new set of clinical trials for different indications, one using inhaled CO and another using a liquid formulation of it, next year. After years of tantalizing scientists in the lab with hints of its potential greatness, the molecule is about to reveal its promise in the clinic. “There’s been enough preclinical data,” says Leo E. Otterbein, who studies the physiological effects of CO at Harvard Medical School. “The time has come—the time is past—to get this into humans and at least see if it works.”
What a gas
It is not surprising that interest in the physiological benefits of CO arose when it did in the 1990s. In 1998, a trio of researchers won the Nobel Prize in Physiology or Medicine for identifying the crucial signaling role of another gas, nitric oxide, in the cardiovascular system. That work established that gases could serve as chemical signals in the body and hinted that there might be other gas signaling molecules—so-called gasotransmitters—to be discovered. In addition to NO, this class of molecules includes CO and hydrogen sulfide. NO is approved by the US Food and Drug Administration to treat respiratory failure caused by pulmonary hypertension in infants. Several drugs that release H2S are now in development for treating inflammatory and cardiovascular conditions. CO-based therapies are seeking their niche, too, but the gas’s bad reputation has made for a rocky path.
To some extent, CO is easier to work with than NO and H2S because it is less reactive toward oxygen and has fewer accessible redox reactions. “CO isn’t a free radical like NO and does not have different protonation states like H2S,” explains Michael Pluth, a chemist at the University of Oregon who studies H2S chemical biology. “So in many ways, the direct reaction chemistry of CO may be simpler than either of these other two gasotransmitters.”
However, CO is haunted by the specter of toxicity. “People learn about carbon monoxide in the context of poisoning,” says Binghe Wang, a medicinal chemist at Georgia State University. That’s a difficult bias to overcome.
Wang and others insist that using CO as medicine poses no special safety concerns. Our bodies make enough of the gas that 1–2% of our hemoglobin is bound up with it normally. In smokers, that number is anywhere from 3 to 20%, depending on the intensity of their habit (it’s not the CO in cigarette smoke that kills: it’s the carcinogens and nicotine inhaled from tobacco products that makes smoking deadly). According to the US Centers for Disease Control and Prevention, exposures that result in more than 50% of hemoglobin bound up with CO can be fatal. Meanwhile, animal studies suggest that a therapeutic dose is somewhere between 6 and 10%. In recent clinical trials of CO, the US Food and Drug Administration limited exposure to 14%. Given those restrictions, staying within a safe and therapeutic range is easily possible, particularly if the molecule is administered in a format that’s more controlled than inhalation, Wang says.
Building the case for benefit
Past work hinted at CO’s positive role in the cell. Epidemiological data from the 1980s, for example, suggested that smokers tend to have lower rates of ulcerative colitis. And neuroscientists speculated in the early 1990s that the gas may be a neurotransmitter—an essential part of cellular signaling. Around that time, Choi’s lab, then at Johns Hopkins University, began testing the physiological effects of CO directly in lab experiments. Several groups had shown that heme oxygenase protects against tissue damage, and Choi wanted to study its effects on lung injury. Heme oxygenase breaks down heme into CO, bilirubin, and iron, but researchers didn’t know which of its three breakdown products was responsible for the tissue protection. Otterbein, working as a graduate student in Choi’s lab at the time, proposed that heme oxygenase’s protective effects stemmed from CO generation and found that exposure to very small doses of CO prevented lung tissue damage in rats and mice.

When Otterbein began to present his findings, however, he ran headlong into a wall of skepticism. “I’ll never forget this one guy,” Otterbein recalls. After he gave his first talk at a conference, a man from the US Environmental Protection Agency stood up in the audience and denounced his work, fuming that his results reflected a nonspecific response and that his work would never be clinically relevant because it proposed to expose people to unsafe levels of CO. Back at Johns Hopkins, colleagues were similarly unsupportive. “My dissertation committee said to pick a different topic,” Otterbein says. “They said, ‘This is stupid; don’t waste your time.’ ”
But he stuck with CO, designing a system that delivered the gas to animals via inhalation to further test its physiological effects. In 2000, Otterbein, Choi, and their colleagues published what became a keystone study for CO therapeutics development. They showed that small amounts of CO quelled inflammation in living animals and identified a signaling pathway through which the effect is mediated. Other labs soon took up the topic. One was a team at Harvard Medical School that transplanted mouse hearts into rats. Using Otterbein’s CO inhalation setup, the researchers found to their amazement that exposing the rats to small amounts of CO protected their organs and prevented rejection.
As research on CO was building in the early 2000s, a team set out to develop small molecules that could deliver it. The researchers integrated CO into the molecular structure of transition-metal complexes that then react to release the gas under conditions present in mammals’ bodies. These so-called carbon monoxide–releasing molecules, or CORMs, allowed researchers to explore CO’s therapeutic effects without dealing with the complications of working with a gas. But although a couple of companies studied them in animal models, they were never optimized for human use because their metal content raised fears that they could be toxic if taken over time.
Meanwhile, a company called Ikaria, which was developing therapies based on all three gasotransmitters, abruptly halted a clinical trial testing whether CO exposure improved outcomes after kidney transplantation. The decision stemmed from changes in company priorities and not from the trial’s results, Otterbein says, and it resulted in the company closing the CO arm of its research. With Ikaria’s change in direction, efforts to take CO to the clinic all seemed to evaporate, Otterbein says. “They all failed, but for reasons unrelated to carbon monoxide.”

Delivery vehicles
Five years ago, when Georgia State’s Wang encountered the prospect of CO as a therapeutic, the field’s forward momentum had stalled. Wang had been studying H2S when he stumbled upon a paper about CO’s anti-inflammatory effects. It piqued his interest, and he dove into the CO literature, emerging convinced of CO’s therapeutic potential. But for such a therapy to succeed, he reasoned, it would have to be turned into a pill or some other type of molecule that was easy and safe to administer. The most obvious option, he thought, was to make a so-called pro-drug—a compound that chemically reacts at the site of treatment to release the bioactive molecule. The metal-based CORMs developed previously are also technically prodrugs in that they undergo a chemical reaction to release CO, but they had not been designed with human drug delivery in mind, since some contain ruthenium or other metals of concern for human consumption. Wang knows a thing or two about drug delivery—he edited a textbook on drug delivery chemistry—so he saw right away that the process of creating a carrier for CO that addressed key properties such as absorption, distribution, excretion, metabolism, and toxicity would involve coming up with some elegant chemistry.
CO is a funny little molecule. The triple bond that ties its carbon to its oxygen is the strongest covalent bond known on Earth; under physiological conditions, the molecule is inert. “CO goes in as CO and comes out as CO,” Wang says. “In the body it undergoes no metabolism.” So getting it to bind to a carrier would be challenging.

What’s more, it’s not subject to the usual tricks of medicinal chemistry. Normally, turning a drug candidate into a prodrug that can undergo a chemical reaction requires tweaking some of the functional groups it carries. But CO doesn’t carry any, so it provides no “handle” to work on, Wang explains. That means that the gas molecule must bond to its carrier compound via the carbon atom and that releasing CO at its intended delivery site would require breaking a carbon-carbon bond. Plenty of reactions will do that, but Wang was hard pressed to think of ones that occur under conditions that exist inside the body. Another restriction in the prodrug’s design is that the carrier molecule can’t latch onto any other partners in the body and can’t interfere with other biological reactions occurring there.
Advertisement
Wang spent a couple of months drawing different possibilities in a notebook. During that time, he thought back to a seminar he was asked to give as a graduate student on so-called extrusion reactions, in which a group of target atoms is cleaved from a molecule through a pericyclic reaction. The more he mulled it over, the more convinced he became that the extrusion reaction chemistry he learned for that seminar almost 40 years ago offered the best chance for success. Using it, he developed a series of organic CO-containing prodrugs that he synthesized with click chemistry.
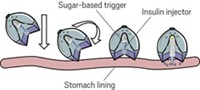
He and his colleagues synthesized some that release CO quickly to deliver a high concentration and others that offer sustained release over several hours or spring CO free at a specific pH so that the treatment won’t be deployed until the molecule passes through the stomach and into the gastrointestinal tract. Some can also target a particular site or organelle in the body, such as mitochondria, or deliver CO along with a second compound, such as a chemotherapy agent. In the latest generation of molecules, soon to be published, the release of CO leaves behind a molecule that is already used as a food additive and is proven to be safe in people, Wang says. The reactions vary, but many involve a CO molecule as a bridge across a cyclic portion: the CO is released via a cheletropic reaction. Others have a CO group that is attached to other parts of a molecule and that then breaks free in response to a stimulus.
Other CO-delivery contenders are bubbling to the surface too. Right around when Wang and his colleagues first began building their CO prodrugs, a pediatric hematologist at Children’s Hospital Los Angeles and the University of Southern California named Edward Gomperts and his colleagues developed a liquid formulation of CO to treat sickle cell disease. Gomperts and his son, pharmaceutical executive Andrew Gomperts, started a company called Hillhurst Biopharmaceuticals and plan to apply for permission from the FDA to test their CO drug in humans early next year. The Gompertses would not disclose the molecular details of their liquid CO but noted that it doesn’t require coaxing the gas to form any ionic or covalent bonds. “We believe this is the right way to deliver carbon monoxide to patients,” says Andrew Gomperts, the company’s CEO. “But you never know what happens in the clinic until you actually do the trial.”
Otterbein, who serves as a scientific adviser to Hillhurst, says that whichever formulation demonstrates an effect in the clinic will be a big boon to CO’s commercial prospects. However, he believes that the future of CO therapies lies in Wang’s molecules or ones like them. The two are collaborating to test Wang’s molecules in animals, and so far results have been promising. But they have yet to pin down exactly which ones to take forward into clinical trials.
Meanwhile, Choi and his colleagues have launched a company called Proterris that aims to deliver CO via inhalation and is also developing reformulated CORMs made from molybdenum and other organometallic and nonmetallic scaffolds. Molybdenum is an essential micronutrient for living organisms and therefore less likely to be toxic than the CORMs of yore—especially in acute indications—and the company believes that it can be safely used in humans. The redesigned CORMs are still being tested in animals, but next year the company plans to relaunch the kidney transplantation trial that was halted by Ikaria a decade ago. Proterris hopes to test the inhaled gas in lung transplantation procedures next year as well. CO’s applications are so broad that Proterris’s CEO, Jeffrey Wager, has no doubt that there’s room for several companies and approaches. “For the sake of public health,” he says, “we really think this needs to see the light of day.”

Alla Katsnelson is a freelance writer. A version of this story first appeared in ACS Central Science: cenm.ag/carbonmonoxide.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter