Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
Covid-19
Chloroquine’s use to treat COVID-19 is backed by US government, but many questions remain
Old anti-malarial drugs have shown mixed results in the small clinical trials conducted so far
by Ryan Cross
March 26, 2020
| A version of this story appeared in
Volume 98, Issue 12
Chloroquine and hydroxychloroquine, a pair of old drugs used to treat and prevent malaria, are the latest compounds to be thrust into the limelight as people tout them as treatments for the novel coronavirus.

On Sunday, March 29, the US Department of Health and Human Services accepted 30 million doses of hydroxychloroquine sulfate from Novartis and 1 million doses of chloroquine phosphate from Bayer. The medicines, which were donated to the Strategic National Stockpile, may be used in formal clinical trials to assess the drug's efficacy in treating COVID-19. The US Food and Drug Administration also issued an emergency use authorization allowing doctors to prescribe the donated drugs to hospitalized teen and adult patients with COVID-19 who are unable to participate in a clinical trial.
The moves follow the publication of studies in February and March suggest that the drugs inhibit SARS-CoV-2, the virus that causes COVID-19, in monkey cells grown in the lab. The drugs are cheap and considered safe, although they can cause severe side effects, including heart problems. President Donald Trump touted the drugs as ready-to-go in a White House briefing on March 19, saying they “could be a game changer” and that “even if things don’t go as planned, it’s not going to kill anybody.”
A flurry of news about the drug followed. A man died and his wife was hospitalized after the couple ingested an aquarium cleaner that contained chloroquine phosphate. Demand for the drug spiked and hospitals began reporting shortages.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe
A small clinical trial in France suggested that hydroxychloroquine helped patients recover from the virus faster, while another small study in Beijing reported that the drug offered no noticeable benefit. Several clinical trials, including one sponsored by the World Health Organization, are expected to put the drugs through more rigorous tests.
Over the past decade, chloroquine has repeatedly popped up as a potential antiviral for emerging pathogens. Studies in 2004 and 2005 identified it as an inhibitor of the original SARS coronavirus. Since then, scientists have conducted several studies that used chloroquine or hydroxychloroquine to prevent or reduce infections of viruses in mice, including the OC43 coronavirus, avian influenza, Ebola virus, Zika virus, MERS-CoV—the coronavirus that causes Middle East respiratory syndrome.
Studies in humans have been less successful, though, and the drugs have never been approved to treat viral infections. In fact, Bayer's chloroquine phosphate, discovered in 1934 and sold as Resochin today, is not approved for any use in the US, although other companies' formulations of the drug are. Despite the lack of good evidence in humans, several drug firms, including Bayer, Novartis, and Teva Pharmaceutical, have pledged to donate millions more doses of the drugs over the coming months, while firms like Mylan announced plans to ramp up production.
Clinical trial rush
Chloroquine has a long history of treating and preventing malaria, which is caused by a parasite that is spread by mosquitoes. Today, its chemically-modified counterpart, hydroxychloroquine, is considered less toxic and is more widely used.
In February, a team of scientists from China’s State Key Laboratory of Virology in Wuhan and its National Engineering Research Center for the Emergency Drug in Beijing showed that low micromolar doses of chloroquine and remdesivir were effective at inhibiting the virus in African green monkey kidney cells, which are often used in virology studies. In a follow-up study, the scientists found that hydroxychloroquine was also effective, although slightly less potent, at inhibiting the virus. Another team found that hydroxychloroquine was more potent than chloroquine in the monkey cells.
Chinese scientists have been quick to act on these studies. The Chinese Clinical Trial Registry lists more than 20 trials using the drugs to treat people with COVID-19, although several of these trials have already been canceled as the virus wanes in China. Studies of chloroquine and hydroxychloroquine for treating COVID-19 are planned or underway in England, France, Norway, Spain, Thailand, and the US.
A team of researchers led by Didier Raoult at France’s IHU Méditerranée Infection published a small study on March 20 suggesting that hydroxychloroquine and the antibiotic azithromycin could pack a powerful punch against the virus. Hydroxychloroquine treatment alone appeared to eliminate the virus in 14 out of 20 people who received the drug for 6 days, compared to only 2 out of 16 people in the control group. Six people who received hydroxychloroquine also got azithromycin, and all of them were deemed “virologically cured” within 6 days (2020 Int. J. Antimicrob. Agents, DOI: j.ijantimicag.2020.105949).
Trump cited the study on Twitter on March 21, saying that hydroxychloroquine and azithromycin together have “a real chance to be one of the biggest game changers in the history of medicine.”
But scientists are quick to point out several caveats. For starters, the study was small and open label, meaning the doctors knew who was getting the drugs. The clinicians only swabbed for the virus in the nasopharynx, an opening behind the nasal cavity and above the throat, meaning the virus could still be in the lungs or elsewhere in the body. Furthermore, six people who received hydroxychloroquine didn’t stay in the study: one left the hospital after 3 days, one quit taking the drug because of nausea, three were transferred to an intensive care unit, and one died.
Preliminary results from a small study in Shanghai also rained on the hydroxychloroquine parade. After 7 days of standard medical care plus hydroxychloroquine, 14 out of 15 COVID-19 patients appeared to be virus-free, while 13 out of 15 people in the control group who only received standard care were also virus-free. The team concluded that the “prognosis of common COVID-19 patients is good”—with or without hydroxychloroquine.
“These potentially conflicting results reflect the limitations of non-blinded, very small clinical studies using regimens that were developed from limited data,” says Peter Madrid, a researcher at the nonprofit R&D firm SRI International. “There is a reason why the clinical testing of drugs is done using randomized, double-blind, statistically powered clinical trials. It is very easy to get excited, and fooled, by small, potentially biased studies.”
Other scientists have a more optimistic view of the two clinical trials. Roberto Cauda, an infectious disease scientist at the Catholic University of the Sacred Heart in Rome, says there are “remarkable differences between the two studies that could explain the different results.” The Chinese study gave patients 400 mg of hydroxychloroquine per day for 5 days, and the French study gave patients 600 mg of the drug per day for 10 days, he notes. The addition of azithromycin in the French study may have prevented additional respiratory infections, and there may have been a synergistic effect with the two drugs, Cauda adds.
Andrea Savarino, a virologist at the Italian National Institute of Health, agrees that the discrepancy in dosing is important. “Although both studies have their own limitations and very limited statistical power,” he says, “at present there is no reason why we should not trust the preliminary results reported by either of them.”
Multiple studies of the drugs are either planned or underway in the US. New York Governor Andrew Cuomo announced that New York state had acquired 10,000 doses of Zithromax (Pfizer’s brand-name azithromycin), 70,000 doses of hydroxychloroquine, and 750,000 doses of chloroquine. Cuomo said trials of the drugs will begin on March 24.
The World Health Organization is sponsoring a large international clinical trial called SOLIDARITY to study six drugs that could be rapidly deployed for the fight the coronavirus, including chloroquine and hydroxychloroquine.
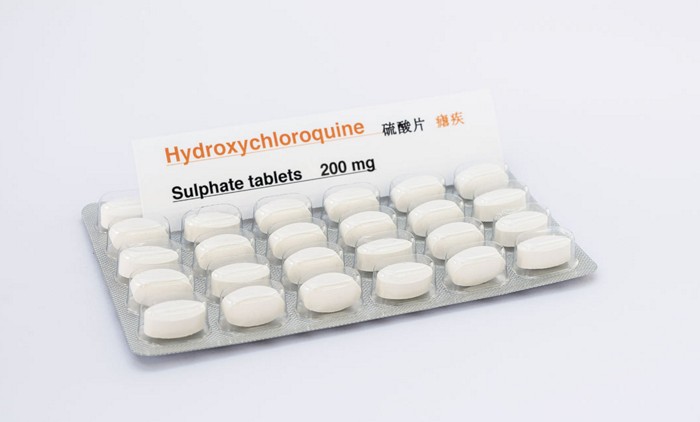
Demand for drugs skyrockets
Demand for the drugs has spiked and they are now in short supply. On March 24, Vizient, a firm that works with hospitals, said it had seen 6,800% and 2,200% increases in orders for chloroquine and hydroxychloroquine, respectively, during the previous week. Most of those orders couldn’t be filled.
Several drug companies are hoping to fill the gap. Rising Pharmaceuticals said it will donate 1 million hydroxychloroquine sulfate tablets in March and April. Bayer announced it will donate 3 million tablets of its chloroquine phosphate drug, called Resochin, to the US government. Teva said it will donate more than 10 million hydroxychloroquine sulfate tablets to US hospitals within a month. Novartis upped the ante and committed to donating up to 130 million hydroxychloroquine sulfate tablets by the end of May, including the 50 million tablets it has on hand.
Companies are ramping up production of the compounds too. Amneal Pharmaceuticals expects to produce 20 million hydroxychloroquine sulfate tablets by mid-April. Mylan said it is restarting production of hydroxychloroquine sulfate tablets at its plant in West Virginia, and expects to make more than 50 million tablets, the first of which will be available in mid-April.
The shortage is worrying people with immune conditions like lupus erythematosus and rheumatoid arthritis who sometimes rely on hydroxychloroquine. But the drug is not a preferred treatment. Sanofi, which sells hydroxychloroquine sulfate tablets as Plaquenil, warns that it should only be taken by these people if they don’t respond well to other drugs with less severe side effects.
The prescription for Plaquenil warns that the drug commonly causes headaches, abdominal pain, blurred vision, diarrhea, headaches, nausea, vomiting, and a loss of appetite. It can also cause hypoglycemia and serious heart conditions in some people including cardiomyopathy, cardiac arrhythmias, and death.
“Overdosing has to be avoided,” says Thomas Dörner, a doctor at Charité–Berlin University of Medicine who has studied the role of hydroxychloroquine in people with immune conditions. And in the interest of patients with immune and rheumatic diseases that rely on hydroxychloroquine, “uncontrolled use of these meds should be strictly avoided.”
Old age and comorbid conditions “may indeed be a limiting factor” in widespread use of chloroquine for treating the coronavirus, Cauda says. Since the data from clinical studies on the drug in COVID-19 patients is meager, Cauda says that “it is necessary to maintain the most caution in the use of chloroquine in all its formulations” until data from the WHO trial and others provide “more robust results.”
Many questions remain
It’s not entirely clear why chloroquine and hydroxychloroquine would treat viral infections, although there are several possibilities. Dörner says the drug possibly “prevents an over-activation of the immune system and therefore permits a more efficient viral clearance.”
Other scientists point to work done with the original SARS virus that suggests chloroquine alters glycosylation, a process of decorating proteins with unique patterns of sugar. The virus’s spike proteins, which it uses to grab onto cells and begin its infiltration, are glycosylated, so interfering with this process may prevent newly created viruses from infecting other cells.
Perhaps the most popular explanation is that the molecules are weak bases, meaning they can raise the pH of normally acidic endosomes—vesicles that the viruses use to sneak into cells. Madrid, who has studied chloroquine and Ebola at SRI International, says chloroquine accumulates in endosomes, which prevents the breakdown of the vesicles and effectively “traps the viruses” inside.
Additional evidence of chloroquine’s relevance comes from academic groups who are finding it pop up in their searches for existing drugs that could be repurposed to fight the virus, although these studies are only lengthening the list of possible mechanisms by which chloroquine acts.
For instance, a large group of researchers at the University of California, San Francisco studied hundreds of possible interactions between coronavirus proteins and human proteins and looked for drugs known to target these human proteins. The coronavirus protein Nsp6 interacted with the Sigma 1 receptor, which is a target of chloroquine. However, the team notes that the receptors bind many non-polar cationic drugs, and they don’t prove that Sigma 1 is related to the drug’s purported efficacy.
Moleculin Biotech, a company developing cancer therapies, said it will test some of its experimental compounds known to target glycolysis and glycosylation to see if they can inhibit the coronavirus. Aldeyra Therapeutics, a firm developing drugs for dry eye diseases, said it will screen its library of reactive aldehyde species inhibitors, which includes molecules that are structurally similar to chloroquine, as potential treatments for COVID-19.
“It is clear that these drugs have broad-spectrum in vitro activities, but this has not yet translated to approved uses for any type of antiviral therapy,” SRI’s Madrid says. “I wouldn’t just rule them out, especially given the lack of any proven effective therapies, but we just need to carefully experiment with them and collect data.”
CORRECTION
This story was updated on March 26, 2020, to correct a reference in the second paragraph to the cells in which chloroquine and hydroxychloroquine were studied. They were monkey cells, not human cells. It was updated on March 30, 2020, to describe the US government's March 29 decision to accept donations of the drug and allow it to be prescribed to some patients, and to note that Bayer's chloroquine phosphate, sold as Resochin, is not approved for any use in the US.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter