Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
DNA-based switch can regulate the activity of a clot-busting enzyme
Engineering an on-off switch into enzymes could expand their therapeutic potential
by Jyoti Madhusoodanan, special to C&EN
November 27, 2018

In the hours after a heart attack, an intravenous dose of the bacterial protein streptokinase can break up blood clots and restore circulation. But it’s not ideal: Like other clot-busting drugs, streptokinase can prove too potent, increasing the risk of hemorrhages. Now researchers have countered this problem by fashioning an on-off switch for the protein out of short strands of DNA. With this switch, the researchers can regulate the enzyme’s clot-busting activity in vitro (J. Am. Chem. Soc. 2018, DOI: 10.1021/jacs.8b10166).
In living systems, enzymes such as streptokinase are only made when specific genetic switches are turned on, and the enzymes’ activities are then closely regulated by small molecules or other proteins. But these regulatory mechanisms are lost when enzymes are isolated and purified for use as drugs, so it’s difficult to control when and where an enzyme will act. That lack of regulation limits researchers’ ability to develop enzyme-based therapies, says M. Reza Ghadiri of the Scripps Research Institute.
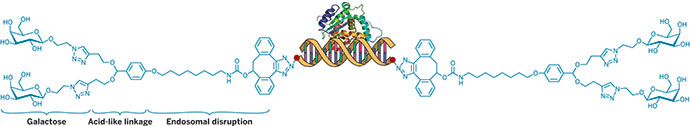
Conventional streptokinase doesn’t digest clots on its own. It acts indirectly, by binding to the blood protein plasminogen and converting it to an active form, plasmin, which then breaks down clot proteins. So Ghadiri and his colleagues used DNA to engineer a regulatory switch into streptokinase that could stop it from activating plasminogen.
The researchers created two versions of the switch. In the more advanced design, they connected a short strand of DNA to streptokinase and added a complementary strand of DNA that carried an inhibitor molecule. Once the complementary strand had annealed to the enzyme’s DNA tether, the inhibitor switched the enzyme off: Streptokinase could still bind plasminogen but was unable to activate it.
To switch streptokinase back on again, the researchers then added another piece of DNA that was complementary to the inhibitor-carrying strand. This removed the inhibitor from the tether, reactivating streptokinase. Placing the inhibitor on a complementary DNA strand instead of binding it to streptokinase directly makes the system more modular and easier to synthesize, says Purba Mukherjee, a postdoctoral researcher in Ghadiri’s lab and the study’s first author.
When the team tested this system on blood clots in vitro, the reactivated enzyme dissolved 87% of fresh clots within an hour, whereas the inactive form digested less than 5% in the same time. By adding regulatory motifs to the sequences of the DNA tether and the complementary strand used to reactivate the enzyme, the researchers could program streptokinase to act only at specific locations or certain times.
Advertisement
The study serves as a proof of principle that the method could also work with antibodies that bind to other enzymes, Ghadiri says. “There could be a whole world of applications if it’s possible to reprogram enzymes to work at precise times and places.”
Future studies will need to test the stability of a DNA tether in living systems, according to Maarten Merkx of Eindhoven University of Technology. Even if a DNA-based system proves unfeasible, peptides or other tethers could work in similar ways, he adds.
“It’s exciting to see how this new study uses DNA’s programmability and its ability to be responsive to inputs to target a therapeutic enzyme,” says Janarthanan Jayawickramarajah of Tulane University. He adds that the paper “highlights how DNA-linked small molecules can be used for a variety of precision medicine applications in the future.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter