Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
Like CBD, but better
Companies aim to treat myriad disorders with molecules akin to cannabinoids
by Britt E. Erickson
August 29, 2020
| A version of this story appeared in
Volume 98, Issue 33

Cannabidiol (CBD) is all the rage in the wellness industry, but most large drugmakers are not interested in a molecule they can’t patent. A few start-ups and niche firms, however, are developing small molecules that are inspired by CBD and other natural products from cannabis plants as potential drugs for a wide array of diseases.
Whether CBD analogs or new chemical entities, these compounds are designed to target particular receptors or have some other function that appears to make them superior to CBD and other cannabinoids in terms of effectiveness for treating pain, inflammation, fibrosis, and many other ailments. Human clinical trials are underway or just wrapping up for some of these drug candidates, while others are still in research or preclinical stages.
CBD reality check
CBD is one of dozens of nonintoxicating cannabinoids produced by cannabis plants. The chemical is commonly extracted from hemp and sold in food and beverages, oil drops, capsules, and cosmetics. Regulators have not reviewed the safety and effectiveness of these products, which are often touted for health benefits, such as promoting relaxation, pain relief, and anti-anxiety effects.
“People say they need CBD to stay healthy or to stay in balance. They put it in their coffee; they put it on their skin,” says Linda Klumpers, partner and cofounder of Verdient Science, a Denver-based R&D consulting firm for cannabis-focused companies. Scientifically, she says, “most of these claims are completely unfounded.”
The one thing that CBD is proven to do is decrease convulsions in patients with certain types of epilepsy. GW Pharmaceuticals developed the drug Epidiolex, an oral solution containing 100 mg/mL CBD extracted from cannabis plants, for controlling seizures in children with rare epileptic disorders. Epidiolex is the only CBD drug approved by the US Food and Drug Administration. The drug hit the US market in 2018.
How CBD works is not fully understood, Klumpers says. “It is actually a pretty dirty drug,” she says, referring to CBD’s ability to influence multiple physiological pathways in animals and humans. Nonetheless, much of the interest in CBD has focused on its effects on the endocannabinoid system, specifically its ability to affect two cell-surface cannabinoid receptors known as CB1 and CB2.
CBD doesn’t actually bind to the CB1 and CB2 receptors. At high doses, CBD affects the endocannabinoid system indirectly by causing other compounds made by the body—endocannabinoids—to bind better to those receptors. Epidiolex is an effective antiepileptic drug because of CBD’s ability to influence CB1 receptors located in the brain.
Increasing potency
A few smaller companies are pursuing drug candidates designed to activate CB2 receptors, which are located throughout the body on immune cells. Activation of CB2 receptors is associated with anti-inflammatory effects.
One of those companies is Massachusetts-based Corbus Pharmaceuticals. Corbus is investigating a compound called lenabasum, which scientists designed to activate CB2 receptors directly.
The company has ongoing Phase III clinical trials for systemic sclerosis and dermatomyositis, plus an ongoing Phase II trial for cystic fibrosis.
Corbus is also collaborating with the Autoimmunity Centers of Excellence at the National Institutes of Health to test lenabasum as a treatment for systemic lupus erythematosus, an autoimmune disease. The NIH is funding and executing the Phase II clinical study, which will enroll about 100 patients.
All the diseases Corbus is targeting are chronic inflammatory diseases, in which “the immune system is constantly on. It cannot stop. It is dysregulated,” says Yuval Cohen, CEO and director of Corbus. People with these diseases are typically very ill, disabled, and have a poor quality of life, he says. The diseases are also life threatening and effective therapies don’t exist, he notes.
Lenabasum binds to and activates the CB2 receptors on immune cells, tamping down the immune system’s response to harmful and foreign substances in favor of anti-inflammatory activity. “Your immune system switches from seek and destroy to heal and rebuild,” Cohen says. He notes that endocannabinoids naturally produced by the body that bind to CB2 do the same thing, but they’re short lived and not particularly effective. The advantage of a pharmaceutical approach is that “you can make compounds that are much more potent than the natural stuff” and more focused on the targeted receptors, he adds.
San Diego–based Emerald Health Pharmaceuticals is also pursuing a drug candidate that targets CB2 receptors. Called EHP-101, the molecule is an aminoquinone derivative of CBD designed to activate CB2 receptors and another group of receptors associated with neuroprotective effects called peroxisome proliferator-activated receptor γ (PPARγ).
The company has initiated a Phase II clinical trial to test EHP-101 for the treatment of systemic sclerosis. The Phase I study ended in September 2019. “We have now started Phase II with only minor delays due to COVID-19 because some clinical sites have shut down temporarily. But we have already gotten two patients enrolled, with more ready to begin screening,” says Emerald Health Pharmaceuticals CEO Jim DeMesa. The company plans to include 36 patients at approximately 30 sites in Australia, New Zealand, and the US. “We will be looking at multiple doses to see what the best dose range is for these patients,” examining safety and tolerability, pharmacokinetics, and preliminary efficacy, DeMesa says.
Emerald Health Pharmaceuticals is also planning to initiate a Phase II study in multiple sclerosis (MS) toward the end of this year or the beginning of next year.
For both systemic sclerosis and MS, CB2 and PPARγ activation are validated targets, DeMesa says. EHP-101 also activates the hypoxia-inducible factor (HIF) pathway, which is also a validated target for MS, he adds. Activation of the HIF pathway modulates the immune response and promotes neuroprotection and nerve regeneration.EHP-101 was designed by modifying CBD with an amino group side chain to promote binding to CB2. A quinone group, which is directly responsible for the PPARγ activation and indirectly responsible for the HIF activation, was also incorporated into the center of the molecule, DeMesa says.
Emerald Health Pharmaceuticals is also investigating a drug candidate based on another cannabinoid, cannabigerol (CBG) for the treatment of the brain disorders Huntington’s disease and Parkinson’s disease. “We’ve done proof of concept in animal models, showing excellent results in two models of Parkinson’s and three models of Huntington’s,” DeMesa says.
The molecule, EHP-102, is derived from CBG, so it inherently binds to different receptors and affects different pathways in the body than CBD-based drugs. It does affect PPARγ, but it does not affect CB2 receptors or the HIF pathway, DeMesa says. EHP-102 is more “focused on neurologic pathophysiology rather than autoimmune or inflammatory processes,” making it more neuroregenerative and neuroprotective than EHP-101, he says.
In the pipeline
A few companies are developing drug candidates inspired by cannabidiol and other cannabinoids.
-
Lenabasum
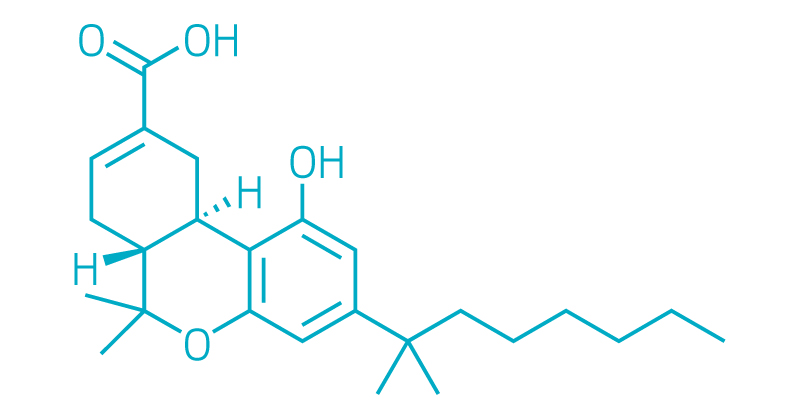
Corbus Pharmaceuticals
Indications: Chronic inflammatory diseases including systemic sclerosis (scleroderma), cystic fibrosis, dermatomyositis, and systemic lupus erythematosus
Development Stage: Phase III clinical trials ongoing for systemic sclerosis and dermatomyositis; Phase II clinical trial ongoing for cystic fibrosis; Phase II clinical trial starting for systemic lupus erythematosus
-
EHP-101
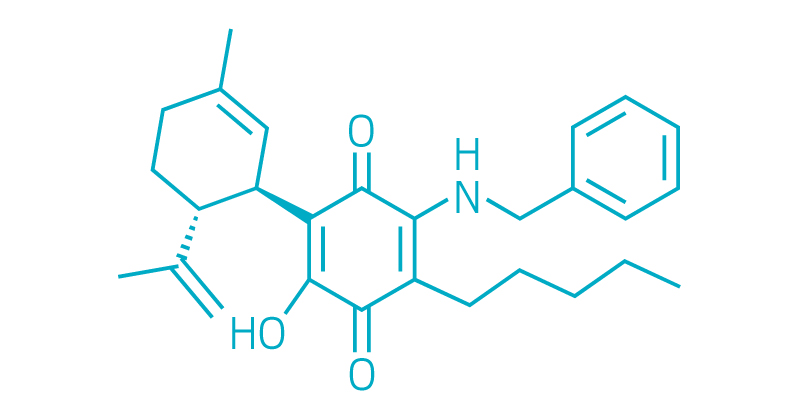
Emerald Health Pharmaceuticals
Indications: Multiple sclerosis and systemic sclerosis (scleroderma); possibly other neurodegenerative, autoimmune, inflammatory, metabolic, and fibrotic diseases
Development Stage: Phase IIa clinical trial started for systemic sclerosis; orphan drug designation in the US and European Union and fast track status in the US for systemic sclerosis
-
THC-Val-HS
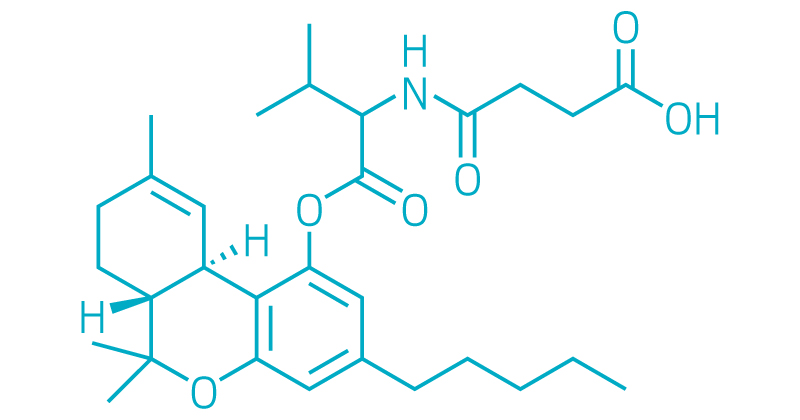
Emerald Bioscience (formerly Nemus Bioscience)
Indications: Glaucoma and other ocular diseases
Development Stage: Preclinical
-
CBD-Val-HS
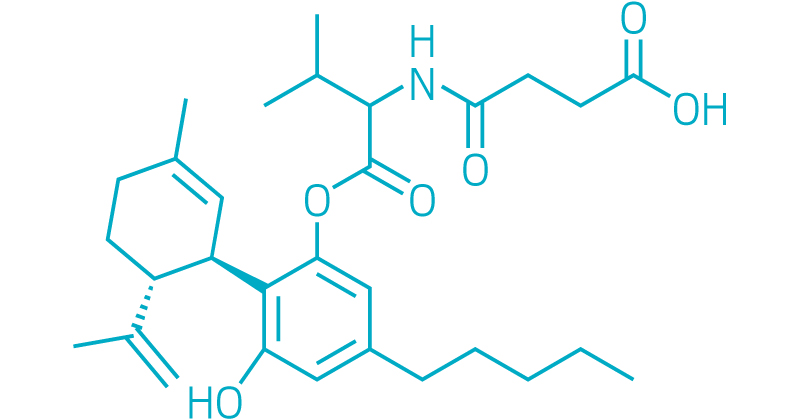
Emerald Bioscience (formerly Nemus Bioscience)
Indications: Infectious diseases
Development Stage: Research; not yet proven to work in animal studies
-
EHP-102
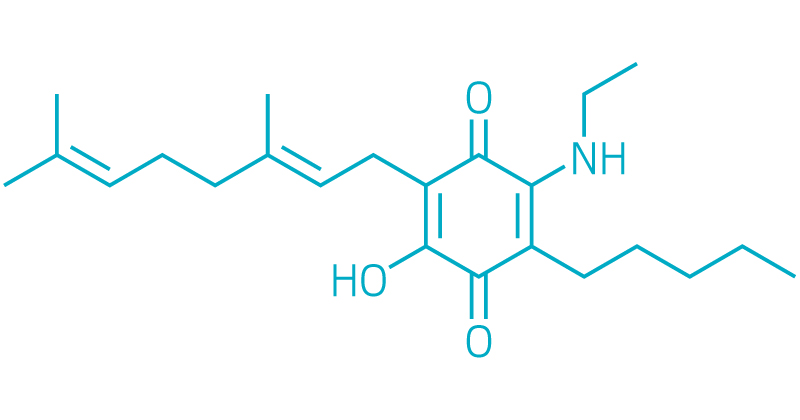
Emerald Health Pharmaceuticals
Indications: Huntington's disease, Parkinson's disease
Development Stage: Preclinical; orphan drug designation in the US and European Union for Huntington's disease
-
KLS-13019
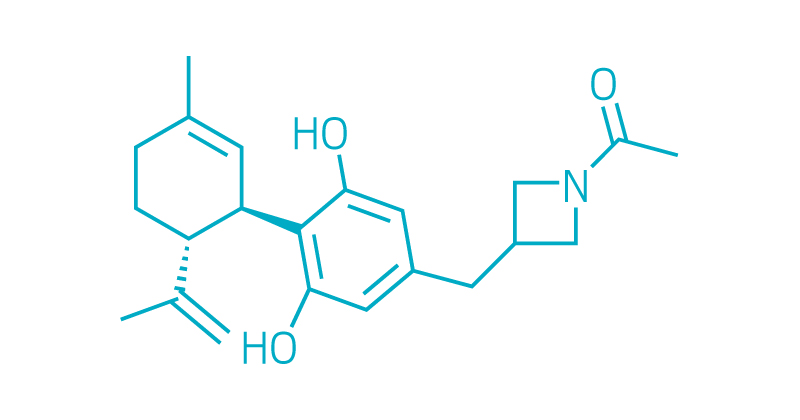
Kannalife
Indications: Chemotherapy-induced nerve and surgical pain; chronic pain from head trauma or stroke; possibly chronic neurodegenerative diseases like Alzheimer's and Parkinson's
Development Stage: Preclinical
-
Tetrahydrocannabinol
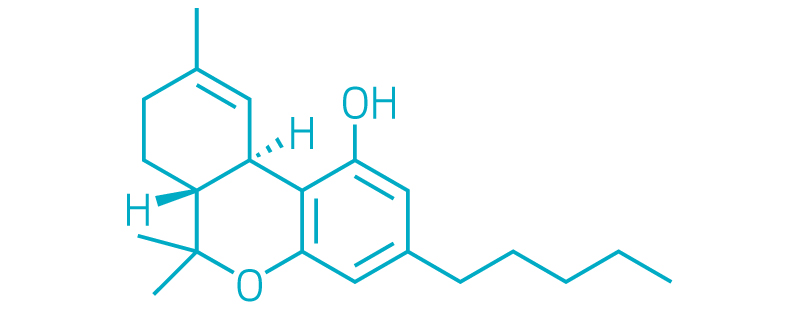
-
Cannabidiol
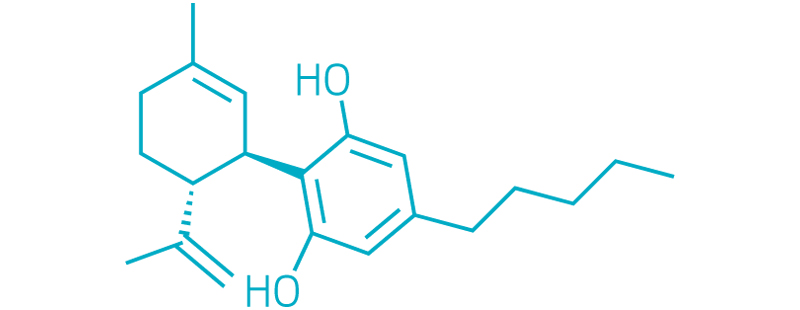
-
Cannabigerol
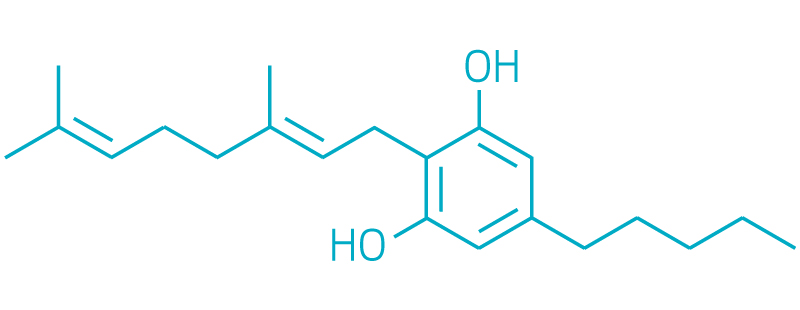
Boosting aqueous solubility
Some companies are enhancing the beneficial effects of CBD simply by increasing its aqueous solubility so that it is more readily taken up by the human body when ingested orally. CBD is a very lipophilic molecule.
Pennsylvania–based Kannalife, for example, is synthesizing new compounds that are structurally similar to CBD but more water soluble. The company’s lead drug candidate is KLS-13019. “We basically modified the pentyl side chain of CBD with the goal of making it more orally bioavailable,” says Kannalife chief scientific officer Bill Kinney. “The strategy was really to increase its aqueous solubility. We wanted to add some heteroatoms like nitrogen and oxygen, which would make it more polar,” he says.
Kannalife became interested in CBD because of its nerve-protective activity, which can be beneficial to patients who have had a stroke or have nerve degeneration disorders, Kinney says. “When we started looking at its properties in our in vitro systems, we noticed that it definitely was effective, but it also had some toxicity” to neurons, he notes.
In addition to increasing oral bioavailability tenfold compared with CBD, KLS-13019 turned out to be about 50 times more potent than CBD and less toxic to neurons, Kinney says.
Kannalife is investigating KLS-13019 for the treatment of chemotherapy-induced nerve damage and surgical pain. “We think it could be broadly useful in other types of chronic pain and injury to hippocampal neurons such as stroke or head trauma,” Kinney says. KLS-13019 could also be beneficial in chronic neurodegenerative diseases like Alzheimer’s or Parkinson’s, but those are much harder to study, he adds.
Preclinical studies in mice, conducted in partnership with pharmacology professor Sara Ward at Temple University, look promising for the treatment of chemotherapy-induced neuropathic pain, Kinney says. That work was funded by the National Institute on Drug Abuse, which is interested in drugs that would replace opiate pain killers and help reduce cravings for opiates.
“Not only were we able to prevent the chemotherapy-induced pain, but we could establish the pain in the first week and then reverse the pain with KLS-13019,” Kinney says. “CBD does not reverse the chemotherapy-induced pain. It can only prevent it.”
Emerald Bioscience (formerly Nemus Bioscience), headquartered in Long Beach, California, is also investigating a drug candidate derived from CBD that has enhanced bioavailability. The molecule, CBD valine hemisuccinate (CBD-Val-HS), was developed by researchers at the University of Mississippi and licensed to the company.
CBD-Val-HS has “potential therapeutic properties as an ocular, analgesic, neurologic, and anti-infective agent, but at this time Emerald Bioscience does not have enough data,” says the company’s CEO Punit Dhillon. Emerald Bioscience plans to investigate CBD-Val-HS for treating infections, such as those caused by methicillin-resistant Staphylococcus aureus, but it is still in the research stage.
The company is also working on a Val-HS derivative of the psychoactive cannabinoid found in cannabis plants, tetrahydrocannabinol (THC). THC-Val-HS has better aqueous solubility than THC and has longer-lasting effects without causing euphoria and intoxication. In preclinical studies, THC-Val-HS worked better to reduce intraocular pressure associated with glaucoma, Dhillon says. Emerald Bioscience now plans to test the drug as eye drops, hoping it will have better ocular-tissue penetration, which is limited with THC. “We are expecting to initiate our first human studies within 12 months” in Australia, Dhillon tells C&EN.
It remains to be seen whether regulatory agencies will eventually approve any of these cannabinoid-based drugs. In the case of lenabasum, if the Phase III trial data are positive, “then we will seek to be approved in the US, then Europe and Japan,” Cohen says. It would be the first synthetic cannabinoid ever approved, the first CB2 agonist drug ever approved, and also the first drug ever approved for systemic sclerosis, he notes.
“Pretty much every big pharma company has some sort of intellectual property on the endocannabinoid system,” Cohen adds. If lenabasum is approved, those companies “can either dust off their programs or they can engage with a company like Corbus and collaborate,” he says. “I have a high degree of confidence that they are not going to stay on the sidelines of an entire new area of biology that is beginning to now get approved drugs.”
Correction
This story was updated on Aug. 31, 2020, to correct the labels on the structures of cannabidiol and tetrahydrocannabinol. They were switched during production.
This story was updated on Sept. 1, 2020, to correct inaccurate information regarding the development stage of lenabasum. The story originally stated that Corbus Pharmaceuticals has completed Phase III clinical trials for lenabasum to treat systemic sclerosis and cystic fibrosis. In fact, a Phase III trial is ongoing for systemic sclerosis and a Phase II trial is ongoing for cystic fibrosis.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter