Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Discovery
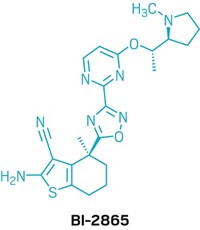
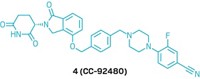
Arvinas unveils PROTAC structures
In 2019 ARV-110 and ARV-471 became the first targeted protein degraders to enter the clinic
by Bethany Halford
April 14, 2021
| A version of this story appeared in
Volume 99, Issue 14

The highly anticipated structures of Arvinas’s clinical candidates ARV-110 and ARV-471 debuted on Sunday at the American Association for Cancer Research meeting. In 2019, these compounds became the first proteolysis-targeting chimeras, or PROTACs, to enter the clinic. Both protein degraders are currently in Phase 2 clinical trials—ARV-110 for prostate cancer and ARV-471 for breast cancer.
Targeted protein degraders are bifunctional molecules that route disease-causing proteins to the proteasome—the cell’s wood chipper, which chops up proteins into their constituent amino acids. One half of the molecule binds to a protein of interest while the other half latches on to an E3 ubiquitin ligase. The E3 ligase then forms a complex that tags the protein of interest with ubiquitin moieties, which in turn signal that the protein should be sent to the proteasome for degradation.
Both of Arvinas’s clinical candidates can be taken orally. ARV-110 targets the androgen receptor, a protein that contributes to the progression of prostate cancer, and ARV-471 targets the estrogen receptor, which can cause certain breast cancer cells to grow unchecked.
Although there are hundreds of E3 ligases, scientists only have identified ligands for a few of them. Both of Arvinas’s compounds bind to the E3 ligase cereblon. And both structures feature the same short, rigid, nitrogen-containing linker—a marked difference from the flexible linkers used in early, academic versions of protein degraders.
Ian Taylor, Arvinas’s chief scientific officer, tells C&EN that the linker was a key innovation in developing these clinical candidates. Although for some targets, long flexible linkers work better, in the cases of the androgen receptor and the estrogen receptor, Taylor says, “the shorter, more rigid ones turned out to be the best.”
Ingo Hartung, director of medicinal chemistry at Merck KGaA, says he’s been looking forward to this reveal for some time. “It’s a real milestone, not only to be in the clinic, but now also to see the structures,” he says. Hartung was surprised that both clinical candidates target cereblon. With two compounds entering the clinic at about the same time, he had expected that one of them would target a different E3 ligase so as not to put all the eggs in the same basket.
Having two PROTACs in the clinic is gratifying, Taylor says. When he joined Arvinas in 2016, many wondered if protein degraders could ever be anything other than niche chemical biology tools. “What we’ve shown is that PROTACs can be drugs. They can work in humans the way that they’ve been working preclinically,” Taylor says, although he notes there’s still a way to go before these compounds are approved. “Now that people can now see the structures and stop guessing about what they are, I think puts a picture to all of that,” Taylor says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter