Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Discovery
Covalent drugs go from fringe field to fashionable endeavor
Designing molecules that make bonds with their biological targets is in vogue
by Bethany Halford
November 9, 2020
| A version of this story appeared in
Volume 98, Issue 43

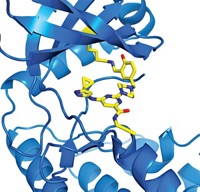
Credit: RCSB PDB/Yang H. Ku | The drug ibrutinib makes a covalent bond to Bruton's tyrosine kinase, an important cancer target.
In brief
Although drugs that make covalent bonds with their biological targets are as old as the pharmaceutical industry, intentionally designing molecules that make such bonds is relatively new. Over the past decade, what was once considered a fringe field has become a mainstay of drug discovery and development, thanks to successful drugs like ibrutinib and new tools that let medicinal chemists demonstrate the selectivity of their covalent drug candidates. Read on to learn about this shift and how chemists are pushing the boundaries of the field.
Enticing atoms to share their electrons—to make a covalent bond, in other words—is at chemistry’s core. But when chemists make drug candidates, they’ve historically shied away from molecules that might engage in this type of bond making with biological targets, preferring instead to use molecules that drift in and out of their protein targets. After all, what’s to guarantee that a reactive compound will make a bond only with an amino acid on its intended target and not one on some other protein in the human body’s complex biochemical soup?
Over the past decade, however, researchers have shifted their thinking about drug candidates that make covalent bonds. After the success of several covalent anticancer drugs, many medicinal chemists are now designing drug candidates that form bonds with their targets. These scientists are finding footholds on proteins that were once considered undruggable; they do this by using analytical techniques that help them zero in on specific amino acids and by developing novel reactive groups that expand the types of amino acids they can target.
Highlights in covalent drugs
Drugs that make covalent bonds to biological targets have been around for more than a century.
1899
Anti-inflammatory drug
- Aspirin (First sold in 1899)
1928
Antibiotic
- Penicillin V (Discovered in 1928)
1989
Heartburn treatment
- Omeprazole
1996
Antibiotic
- Fosfomycin
1997
Blood thinner
- Clopidogrel
2003
Anticancer drug
- Bortezomib
2009
Antidiabetic drug
- Saxagliptin
2013
Anticancer drug
- Ibrutinib
2018
Anticancer drug
- Dacomitinib
2019
Sickle cell disease treatment
- Voxelotor
Note: Dates indicate first approval by US Food and Drug Administration unless otherwise noted. Reactive groups are shown in red.
Highlights in covalent drugs
Drugs that make covalent bonds to biological targets have been around for more than a century.
Aspirin
Anti-inflammatory drug
First sold in 1899
Penicillin V
Antibiotic
Discovered in 1928
Omeprazole
Heartburn treatment
1989
Fosfomycin
Antibiotic
1996
Clopidogrel
Blood thinner
1997
Bortezomib
Anticancer drug
2003
Saxagliptin
Antidiabetic drug
2009
Ibrutinib
Anticancer drug
2013
Dacomitinib
Anticancer drug
2018
Voxelotor
Sickle cell disease treatment
2019
Note: Dates indicate first approval by US Food and Drug Administration unless otherwise noted. Reactive groups are shown in red.
Drugs that make covalent bonds to their targets certainly aren’t new. Aspirin’s reactive acetyl group gets transferred onto serines in certain enzymes, preventing the enzymes from making molecules that lead to inflammation and clotting. Of course, no one knew that in 1899, when Bayer started selling the drug. Even widely used contemporary drugs like the heartburn pill Prilosec (omeprazole) and the blood thinner Plavix (clopidogrel) act by a covalent mechanism that wasn’t discovered until after they were in development. It wasn’t engineered into the molecules.
“If you look back over 100 years, some of the most important medicines advanced have a covalent mechanism of action. I think that was underappreciated until the early 2000s,” says Juswinder Singh, founder and chief scientific officer at Ankaa Therapeutics. “People didn’t realize the importance of the bond that was being formed,” he says. “The power of covalency is that you’ve got a small molecule that essentially silences the drug target.”
Taboo to trendy
A noncovalent, or reversible, drug slips in and out of its target, a disease-linked protein. But a covalent drug bonds to the protein target, shutting it down. That protein won’t be active again until the body resynthesizes it—a process that can take days. That means that doctors don’t have to give the drug as often and can give it in lower doses.
Although those qualities sound appealing, designing covalent drug candidates was considered a fringe idea as recently as 2006. That’s when Singh cofounded Avila Therapeutics, a company dedicated to making covalent anticancer drugs. At the time, drugmakers worried about creating compounds that, in theory, could form bonds with other proteins in the body and cause dangerous, off-target effects. They also thought immune cells might see the drug-bound proteins as foreign and potentially trigger an immune response.
Margaret Chu-Moyer, vice president of research and head of chemistry, characterization, and technology at Amgen, says drugmakers saw how a class of covalent drugs called DNA-alkylating agents, which includes the cancer therapy cisplatin, reacted widely. “They were not only toxic to tumor cells, which is what you wanted; they were like a bomb going off throughout the body,” because of their toxic reactions in other cells too, she says. With that context, it was tough to think about designing covalent drugs that would also be safe, she says.
In fact, Chu-Moyer says, when she started as a medicinal chemist in the 1990s, she avoided making molecules with reactive handles or molecules that could be metabolized into compounds with reactive handles. “It was verboten, almost, to think of these in a prospective manner,” she says.
Singh adds that many drugmakers thought covalent drugs simply weren’t necessary. With rational drug design, he says, medicinal chemists thought they could solve every problem with a reversible drug. “I think it’s still the case that a lot of people believe that,” he says. But now there are plenty of data showing the problems covalent drugs can solve.
“The drug that really put covalent inhibitors on the map is ibrutinib,” says Dan Erlanson, vice president of chemistry at Frontier Medicines, a company that’s taking a covalent approach to drug discovery.
Ibrutinib, which is marketed as Imbruvica, covalently inhibits Bruton’s tyrosine kinase (BTK), an enzyme that is active in certain cancer cells. The drug is one of several covalent BTK inhibitors that researchers began pursuing in the first decade of the 2000s—Avila’s BTK inhibitor was bought by Celgene in 2012. Along with EGFR inhibitors, they were among the first drug candidates to be designed to act covalently.
Ibrutinib was originally designed at Celera Genomics in 2005 as a tool for studying the biology of BTK. Celera sold the compound in 2006 as part of a package deal to Pharmacyclics, which took the molecule through clinical development.
“Part of the reason ibrutinib caused people to take note is because it had been dismissed by a lot of people in pharma,” Erlanson says. Many looked at the structure and thought it wouldn’t be selective. But the US Food and Drug Administration approved the compound to treat mantle cell lymphoma in 2013, and it is now used to treat five other types of cancer too. “That caused people to reassess their assumptions,” Erlanson says.
Another consideration: ibrutinib brings in a lot of money. Its success prompted AbbVie to pay $21 billion for Pharmacyclics in 2015.
Roman Fleck, CEO of Janpix, a biotech firm working on covalent inhibitors, says he and his colleagues see ibrutinib as a benchmark against which they judge their drug candidates. “It was the first molecule that validated in a big way that covalent inhibition is worthwhile—at least in oncology,” he says.
Designing from scratch
Since 1990, the FDA has approved 35 drugs with a covalent mechanism of action, according to data compiled for C&EN by CAS, a division of the American Chemical Society (ACS publishes C&EN). Of those approved in the past decade, 13 had cyclic shapes and scaffolds that had not appeared in any previous FDA-approved drugs. That’s a spike in novelty compared with covalent drugs with novel shapes and scaffolds approved by the FDA in the first decade of the 2000s and in the 1990s.
The boost in structural novelty points to another shift in the area of covalent drugs. Chemists designing covalent kinase inhibitors from 2000 to the early 2010s took molecules that bound reversibly to their targets and outfitted them with a reactive group, like an acrylamide or a chloroacetamide, that could latch on to an amino acid in the target.
While medicinal chemists still use that strategy, scientists are now able to find covalent inhibitors without starting from a molecule that already binds to the target reversibly. Instead, they screen their targets with libraries of small reactive fragments to find ones that make covalent bonds. Those become the starting point for designing covalent inhibitors that can latch on to targets that don’t have deep pockets for a molecule to bind within—targets once considered intractable, like KRAS G12C.
A surge in interest

KRAS is a key protein involved in the signaling processes that make cells divide and proliferate. But KRAS and its family members, mutants of which are found in 30% of cancers, are smooth like cue balls. Drug developers spent decades trying—and failing—to find a good toehold on the proteins.
In 2013, a team led by Kevan Shokat of the University of California, San Francisco, found a possible way to covalently inhibit one of those mutants, KRAS G12C, in which the glycine at the peptide’s 12th amino acid has been swapped out for a cysteine. Shokat discovered that the sulfur in KRAS G12C’s mutant cysteine could act as a nucleophile and covalently latch on to a small-molecule electrophile. And because that mutation is present only in cancer cells, regular KRAS would be unaffected.
“When you’re doing reversible binding, you can take advantage of van der Waals interactions and salt interactions and water,” Shokat says. “But when you’ve got a nucleophile and an electrophile, it’s a reaction, so it’s got a much steeper transition state. You’ve got to get everything right.”
In the case of KRAS G12C, Shokat says, the key was that the acrylamide electrophile the researchers used was perfectly poised to react with the cysteine, with an assistive tug from a nearby lysine. When they tried a similar strategy with KRAS G13C, a mutant in which a glycine just one position further along the protein chain is modified to a cysteine, they couldn’t get anything to work. “It’s probably because it’s too far from the lysine,” Shokat says.
That work inspired a number of companies to make compounds that latch on to that cysteine. “I think KRAS represents a real change in the way that people looked at covalent inhibitors,” says Victor Cee, vice president of chemistry at Oncovalent Therapeutics. Before joining Oncovalent, Cee worked at Amgen on the first KRAS G12C inhibitor to enter clinical trials, sotorasib. Cee says the KRAS G12C inhibitors that have gone into clinical trials came from screening for and then iteratively optimizing molecules that could react with cysteine.
Chemists didn’t understand if they were optimizing the molecules’ binding affinity or their rate of reaction, Cee says, and successful KRAS G12C inhibitors are “oddball molecules” compared with the kinase inhibitors that had been retrofitted with a reactive handle. The drugs that eventually went into the clinic generally don’t have a strong affinity for KRAS G12C, but when they do bind, they react quickly with that cysteine on the protein. So chemists don’t need to get a lot of their covalent inhibitors onto the target as long as they react quickly during a chance encounter.

“This opens up a whole new world of targets for covalent inhibition,” Cee says. A huge family of proteins is considered undruggable because it’s impossible to get a high enough concentration of drugs onto a target to effectively silence it, he says. The KRAS work shows there’s a different way.
Larry Burgess, head of chemistry at Vividion Therapeutics, remembers reading the work from Shokat’s team when it came out. At the time, Burgess was executive director of drug discovery at Array BioPharma, and he and the company’s chief scientific officer decided that day that they had to go after KRAS G12C. They’d already gotten comfortable with the idea of covalent drugs, he says, through earlier work to modify reversible kinase inhibitors. After seeing Shokat’s strategy, they wanted to see if they could create a drug using a de novo approach. The company ultimately teamed up with Mirati Therapeutics to develop MRTX849, another KRAS G12C covalent inhibitor that is in clinical trials.
“That experience really solidified for me that covalent drugs are the way. Not only are they a tool in the toolbox; they are a paradigm unto themselves that really offers a lot of advantages,” Burgess says.
Progress with proteomics
So drugmakers had figured out how to design covalent inhibitors. They’d shown that they could be effective drugs. And they’d used them to go after some challenging targets.
But there still remained the question of selectivity.
Would you develop a covalent drug only to find out late in development that it has an off-target effect you didn’t know about and can’t work around? “In every project, that is the biggest fear,” Cee says.
Those fears have largely subsided, thanks to proteomic screening, according to Burgess and Cee. This technique allows drug developers to look at all the proteins expressed by a cell or an organism and see if their candidate compound will, for example, latch on to only a specific cysteine or if it will make bonds to cysteines in other proteins as well. It is essentially a competition experiment in which scientists expose a proteome—all the proteins in a biological system—to a small molecule that covalently binds to a specific amino acid on a protein of interest. They then throw in a probe that would modify that amino acid indiscriminately and use mass spectrometry to see which proteins have made covalent bonds to the small molecule instead of the probe.
Advertisement
“Before the rise of these high-throughput proteomic techniques, it was kind of a shot in the dark if I said, ‘I’m going to make a covalent inhibitor,’ ” Cee says.
Proteomic screening “gives people confidence that they’re not going in blind,” says Benjamin F. Cravatt of Scripps Research in California, who pioneered the technique and cofounded Vividion in 2014 to use the method for drug discovery. “Our goal all along was to try to demystify the process of covalent ligands in drug discovery, to try to make it more of a science with data that can drive decision-making,” Cravatt says.
In the late 1990s, his lab started looking for amino acid residues in the proteome that are good nucleophiles and can latch on to small electrophiles—precisely what someone developing a covalent drug would want to know. The technique is mostly used to look for nucleophilic cysteines and serines, but it can be applied to other residues, too, like lysines and tyrosines. As high-throughput mass spectrometry–based methods became more advanced, Cravatt says, “it was clear that you can actually make a pretty rigorous science out of covalent-ligand drug discovery, probably a more rigorous science than you can make out of reversible-ligand discovery.” That’s because the mass spectrometry can directly detect when a bond has been made, but it can’t do the same for reversible interactions.
Cravatt says he encountered a lot of skepticism when he first suggested the technique to pharmaceutical companies a decade ago. But since then, drugmakers have used it to go back and determine whether marketed covalent drugs and clinical candidates are selective for their protein targets. And they’ve been using proteomic screening as a tool in current campaigns. Consequently, Cravatt says, “a lot of the drugs that are now being developed are way more selective than the ones that have already been approved.”
John Tallarico, who heads chemical biology and therapeutics at the Novartis Institutes for BioMedical Research in the US, agrees. “Over and over, I’m surprised with how clean these molecules are. I’m not saying they only interact with one target, but it’s not hundreds; it’s tens of targets sometimes, which is fantastic.”
Finding footholds
Using proteomics and reactive fragment screening to go after targets that were once considered undruggable is an area that’s hotly pursued by many companies, including Novartis, Vividion, and Frontier Medicines, a start-up cofounded by Daniel Nomura, a professor at the University of California, Berkeley, who studied with Cravatt as a postdoctoral fellow.
Over 90% of proteins in humans are considered undruggable, Nomura says. “I would argue that’s one of the biggest bottlenecks in modern drug discovery.” But, he says, combining proteomic screening and approaches for discovering covalent small molecules, like using libraries of small reactive fragments to find reactive residues, has “enabled us to tackle areas of the proteome, particularly the undruggable proteome, in ways that we couldn’t access before.”
In collaboration with Tallarico and others at Novartis, Nomura’s lab recently reported it was able to find a small molecule that covalently binds to MYC, a transcription factor, or gene-reading protein, that promotes cell growth and proliferation. Scientists have long considered MYC to be a key driver of cancer, but, like most transcription factors, much of the protein is disordered. “There’s no obvious pocket on this for us to stick a small molecule in, even as a tool,” Tallarico says.
Using proteomic screening, Nomura and coworkers found a cysteine within a disordered region of MYC that they realized they could use to grasp a molecule. They then screened a library of small molecules and found one that targets this intrinsically disordered region, destabilizes MYC, and leads to its destruction.
Tallarico says the small molecule is an interesting tool, but he doesn’t think it will become a drug. Nomura says his team is trying to repurpose the molecule as a starting point for a protein degrader, a bifunctional molecule that binds to both a protein of interest and an enzyme that helps tag the protein for breakdown.
Researchers at Janpix have also had some success using covalent inhibitors to block transcription factors, in this case two proteins related to blood cancers, STAT3 and STAT5. The company has been working with University of Toronto Mississauga medicinal chemist Patrick Gunning on a family of small molecules targeting STAT3 and STAT5. Not only do the molecules covalently bind to these targets, but in the case of STAT5, the inhibitors also unravel the protein so that it gets degraded, Janpix’s Fleck says. Janpix is in the final stages of picking which molecule to pursue as a clinical candidate.
Ties that bind
The Janpix molecules also stand out because they have an unusually reactive handle. Ibrutinib, sotorasib, MRTX849, and many other covalent drugs and drug candidates use an acrylamide as their electrophilic handle, which gets attacked by cysteine nucleophiles.

“Acrylamides are getting to be really popular because people have figured out a lot of the rules for their reactivity and how to modulate that reactivity effectively,” Oncovalent’s Cee says. “They’re the Goldilocks electrophile.” Acrylamides also have a history of success, and they’re not difficult to add to a molecule later in a synthesis. “If you can use an acrylamide, you would,” Cee says.

In contrast, the reactive handle on Janpix’s molecules is a pentafluorobenzene sulfonamide. Cysteines go after this handle too, latching on to the para position of the pentafluorobenzene and displacing the fluoride there. The molecules are outfitted with other groups that encase the reactive pentafluorobenzene and spring open only when they encounter STAT3 and STAT5, Fleck explains. This reactive electrophile is new in covalent drugs, “so people are unfamiliar with it, and people may have reservations about it,” he says. “But an acrylamide could not be shielded in the same way.”
There are already a few approved drugs that feature reactive handles and aren’t based on acrylamides—for example, the anticancer drug bortezomib uses a boronic acid to bind to a threonine, and the antibiotic fosfomycin uses an epoxide to target a cysteine. But they are exceptions rather than general classes that medicinal chemists can use.
Medicinal chemists who want to target amino acids other than cysteine are going to have to look for reactive groups beyond acrylamides, says Matthias Gehringer, a medicinal chemist at the University of Tübingen who is studying new reactive groups for covalent inhibitors. The conjugate addition chemistry that works so well to link cysteine’s sulfur to the acrylamide isn’t suited to other amino acids, he says.
Determining how to create reactive small molecules that selectively latch on to amino acids like lysine, tyrosine, and aspartate is going to take creative chemistry, UCSF’s Shokat says. “Whenever I go to give talks at chemistry departments and have lunch with the students, I always tell them, ‘We need reactions that work in water and attack aspartate,’ ” he says. That’s because the KRAS that drives pancreatic cancer has a mutant aspartate. Finding a molecule that could selectively lock on to that amino acid, Shokat says, “would be fantastic.”
Several academic chemists are developing new reactive groups for amino acids beyond cysteine. UC Berkeley chemists Christopher J. Chang and F. Dean Toste have used oxaziridines that latch on to methionines in proteins, for example.
The sulfur(VI) fluoride exchange, a type of click chemistry developed by K. Barry Sharpless at Scripps Research in California, offers one way medicinal chemists can tack small molecules on to other amino acids, including tyrosines, lysines, serines, histidines, and threonines. Chemical intuition suggests that the sulfonyl fluorides and sulfuramidimidoyl fluorides developed in Sharpless’s lab wouldn’t be very selective, but these substituents have proved otherwise. They hook up with an amino acid only when the protein environment is just right.
This feature prompted the lab to nickname these groups “sleeping beauties.” Only when the molecules encounter the right amino acid prince will they awaken for the key bond-making event. Sharpless says they are still trying to establish what makes the perfect protein environment for the sulfur(VI) fluoride exchange to occur. He suspects there must be a positively charged amino acid nearby that pulls the fluoride away so that the sulfonyl or sulfuramidimidoyl makes a covalent bond to the amino acid.
As they develop new reactive electrophilic handles, chemists have also created molecules that form covalent bonds reversibly—that is, they can be broken and remade at various sites on a protein target. Voxelotor, a sickle cell disease drug developed and sold by Global Blood Therapeutics as Oxbryta, features an aldehyde reactive handle that reversibly attaches to the N-terminus of hemoglobin to prevent it from polymerizing in red blood cells.
Advertisement
“The chemistry is as old as organic chemistry,” UCSF’s Jack Taunton says of reversible covalent inhibitors. “And applying reversible covalent approaches to drug discovery is also very old.” The antidiabetic drug saxagliptin, approved by the FDA in 2009, uses a nitrile to reversibly bind to a catalytic serine on a key protease.
But reversibly binding to noncatalytic residues is relatively unexplored, Taunton says. His lab has made reversible covalent inhibitors using cyanoacrylamides as electrophiles. He notes that a cyanoacrylamide is the key electrophile in rilzabrutinib, a BTK inhibitor that was developed by Principia Biopharma and is currently in late-stage clinical trials for immune-mediated diseases. The reversibility of the covalent bond lets the compound attach and detach from cysteines in various kinases at different rates, Taunton says. Although the details of rilzabrutinib’s selectivity haven’t been disclosed, the compound seems to spend more time on BTK than it does on other kinases.
While much of the innovation in designing covalent drugs has been for oncology, medicinal chemists are using covalent inhibition for other diseases too. The coronavirus pandemic has brought an explosion of work to develop covalent inhibitors of the main protease of SARS-CoV-2. This protease’s active site has a cysteine that’s essential for its activity and therefore a good nucleophilic target for either reversible or irreversible covalent molecules.
Part of the COVID Moonshot project is devoted to screening small fragments that make covalent bonds to that key cysteine with the goal of using that affinity to create drugs. Similarly, Novartis’s Tallarico says the company is working on covalent inhibitors of SARS-CoV-2. And Pfizer recently announced that its SARS-CoV-2 antiviral, which is a reversible covalent inhibitor with a hydroxymethyl ketone electrophile, has entered Phase 1 clinical trials.
As the boom in covalent drugs continues, researchers say there’s still plenty of room for innovative chemistry. Whether it’s making new reactive groups, expanding the libraries that are used to screen possible targets, or establishing footholds on proteins previously thought to be undruggable, chemists have a valuable role to play. “I think we’re at a stage now where covalent ligands in drug discovery are here to stay,” Scripps’s Cravatt says. “I think many companies and many academic labs would prefer a covalent ligand over a noncovalent ligand, which is amazing to say, because 10 years ago that would have been blasphemy.”
Correction
This story was updated on Nov. 9, 2020, to correct the number of FDA-approved covalent drugs and details about their novelty. The FDA approved 35, not 32, of these drugs since 1990, and the 13 with novel shapes and scaffolds are a selection of those approved in the past decade, not all the ones approved in that decade. The novelty is based on an analysis of cyclic compounds.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter