Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Discovery
Novartis research head Bradner outlines a recalibrated drug engine
A portfolio overhaul comes as the research organization homes in on the toughest drug targets
by Lisa M. Jarvis
November 25, 2018
| A version of this story appeared in
Volume 96, Issue 47

A month after revealing a significant pruning of its pipeline, Novartis is providing details about the two-year evolution of its research agenda. In an interview with C&EN, Novartis Institutes for BioMedical Research (NIBR) President Jay Bradner explains the tough decisions and the renewed focus of the pharma giant’s R&D engine.
“Outside our walls, there’s a footrace for the lowest-hanging fruit,” Bradner says. Novartis might participate in that race on occasion, he concedes, but his research organization is squarely focused on first-in-class molecules or drugs that offer more than just an incremental improvement over existing treatments. As one of the rare big pharma companies devoting significant time and money toward early research, he says, “we have the luxury to reach for the highest-hanging fruit.”
The portfolio review that took place between July and September aimed to weed out programs that didn’t jibe with that lofty ambition, belonged to a therapeutic area Novartis has decided to abandon, or were ones where the business rationale simply didn’t add up. In the end, Novartis cut 20% of its portfolio, or 90 projects. It also ended research in infectious diseases.
It is the first major portfolio overhaul under the watch of Bradner, a chemical biologistwho in early 2016 left Harvard Medical School to lead NIBR’s 6,000 researchers.
That research organization has trained its sights on several risky, but potentially highly rewarding, areas of science. “We have made a significant investment and have focused recruitment in the field of chemical biology, believing that this is a field of science that has matured to the point of promising new types of drug molecules to overcome long-standing challenges in discovery chemistry.”
That has meant organizing teams of scientists around a few key concepts that include next-generation DNA-encoded library chemistry, small molecules that modulate RNA splicing, covalent chemoproteomics, targeted protein degradation, and what Bradner calls “a new brand of phenotypic drug discovery.”
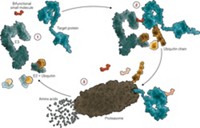
Bradner himself leads the sizable team working on targeted protein degradation, a way of using complex small molecules to tag errant proteins for the cellular trash bin. Although it’s unusual for a major drug company’s R&D head to take such a hands-on approach, Bradner has a special attachment to the field: It was a focus of his academic lab, work that led to one of the first protein-degrader-focused biotech companies, C4 Therapeutics.

Moreover, the method of breaking down proteins, rather than simply blocking their activity, aligns with Bradner’s mandate that NIBR scientists take on proteins that have long eluded drug hunters. “The community should expect that the proteins targeted by protein degradation are otherwise intractable,” he says, noting that NIBR has worked on historically inaccessible targets like nonenzymatic scaffolding proteins and transcription factors. Although he declined to provide a detailed timeline, Bradner expects Novartis and others will have their first protein degraders in the clinic in 2019.
Another emerging area that has gathered momentum is designing small molecules to modulate splicing of RNA. The company already has one such compound, LMI070, in early clinical trials to treat spinal muscular atrophy and has since worked internally and with collaborators to explore the idea of whether it’s possible to directly drug RNA. “If RNA is druggable, then nothing is undruggable,” Bradner says.
Advertisement
Bradner says that of the 340 drug discovery projects that survived the pipeline review, small molecules targeting RNA and protein degradation are both “well represented.”
As for the molecules that Novartis terminated, Bradner believes many will find homes outside Novartis. Over the past two years, Novartis has built a group within its business development unit that is looking at opportunities to license products like the ones recently trimmed from the pipeline. That could include conventional licensing deals like the one reached last month with Boston Pharmaceuticals, which bought the rights to three antibiotics, or enabling new company formation like with QED Therapeutics, which launched in January to develop a fibroblast growth factor receptor inhibitor that Novartis had discontinued.
Now, with even more programs collecting dust, “we will be working through 2019 to start focused new biotech companies and/or partner with existing discovery units,” Bradner says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter