Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nanomedicines
Sprayable nanomedicine restores function after heart attacks in mice
Gold nanoparticles and peptides help damaged heart tissue recover the ability to pump blood
by Alla Katsnelson, special to C&EN
March 10, 2022
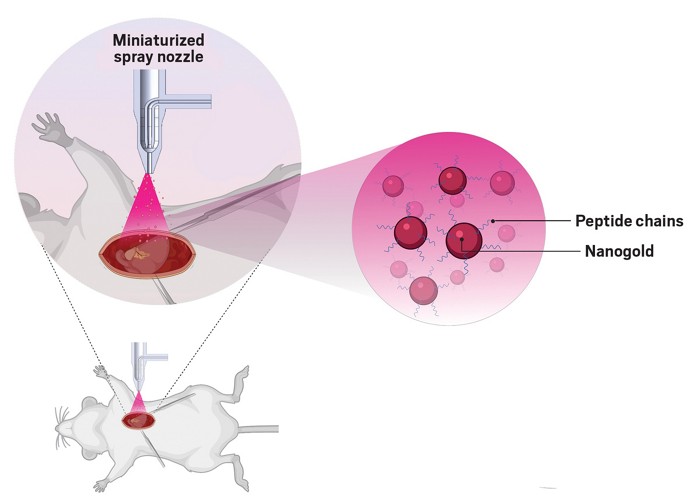
The heart is a powerful but delicate organ: after a heart attack, damaged tissue loses its ability to contract effectively and to conduct electrical impulses that help it beat, potentially leading to heart failure. A spray-on therapy containing a mixture of gold nanoparticles and synthesized peptides can help restore this lost heart function in animals, a new study reports (ACS Nano 2022, DOI: 10.1021/acsnano.1c08890).
“There are currently no treatments to recover the functionalization of the heart,” says Marcelo Muñoz, a biological chemist at the University of Ottawa Heart Institute who led the work.

Many studies have suggested that gold nanoparticles can promote heart tissue recovery. Because they are metallic, they improve the organ’s conductivity, and they also seem to tamp down the immune response that follows tissue injury, Muñoz explains. But when injected into the bloodstream, they tend to clump together, which keeps them from spreading in the heart tissue and shuts down their therapeutic effect. And in high concentrations, they are toxic.
To help the nanoparticles embed into heart cells, Muñoz and his colleagues mixed them with specially engineered, positively charged peptides. The peptides they’ve designed have a spiderlike structure, with multiple “legs” that encircle the surface of the nanoparticles.
In past studies, such peptides promoted functional recovery after a heart attack but are normally quickly degraded by inflammatory enzymes that flood the damaged heart tissue. Here, researchers observed that the nanoparticles help prevent the peptides’ degradation, while the peptides in turn stabilize the nanoparticles by keeping them from aggregating. This synergy makes it possible to use a low concentration of particles in the treatment, which guards against the particles’ toxicity.
Researchers have tried targeted approaches for getting gold nanoparticles into heart tissue such as patches that release them onto the heart’s surface, but differences in the shape and size of the organ across individuals prevents patches from having good adhesion. Instead, Muñoz and his colleagues developed a microspray nozzle that can deliver the therapy onto the heart.
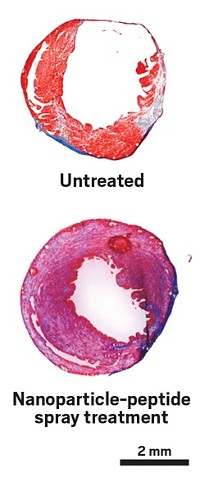
The researchers applied the spray-on therapy in mice, delivering it 1 week after a heart attack. It restored the contractility and conductance in the damaged tissue to healthy levels, allowing the heart to pump 2.4 times as much blood as untreated hearts.
“Mouse hearts are tiny,” Muñoz says, so to spray them, the researchers had to conduct open heart surgery. In people, though, it should be possible to spray the substance onto the heart using a micronozzle delivered through a catheter, he says. But before then, he and his colleagues plan to test the system in larger animals and to identify better peptides that would allow them to decrease the therapy’s nanoparticle concentration further still.
The therapy’s efficacy in mice is promising, but further studies will need to demonstrate that it’s compatible with human tissue, says Stephanie Willerth, a biomedical engineer at the University of Victoria who wasn’t involved in the work. “The use of a biomaterial-based spray gets around some of the major limitations of the use of cardiac patches for heart repair.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter