Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceutical Chemicals
Chemists think outside the box to craft tricky cubanes
Using cubanes in place of benzene rings could help fine-tune the properties of drug candidates
by Mark Peplow, special to C&EN
April 24, 2023

Whoever said chemistry is for squares may have had a point. A team led by David W. C. MacMillan of Princeton University has developed a handy set of synthetic methods to make cube-shaped molecules called cubanes (Nature 2023, DOI: 10.1038/s41586-023-06021-8).
A cubane contains eight carbon atoms, one at each corner of the structure. It is almost the same size as benzene, and although the molecules are chemically quite different, both can bear substituents oriented at similar angles. This means that replacing a benzene group in a drug molecule with a cubane can sometimes improve the drug’s solubility or metabolic stability without affecting its biological activity.
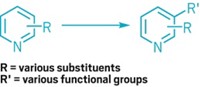
But two problems have made it hard to use cubanes as benzene surrogates, or bioisosteres, in medicinal chemistry: they are difficult to make and difficult to modify.
The most common commercially available cubane is synthesized in eight steps and carries two ester groups on opposite corners, a pattern known as 1,4-substitution that mimics a para-substituted benzene ring. But it takes eight more steps to create 1,3-substituted cubanes, which feature two groups on the same face of the cube, or 1,2-substituted cubanes, which have two groups on the same edge. These structures would mimic meta- and ortho-substituted benzenes, respectively. Meanwhile, the cross-coupling reactions often used to modify organic molecules typically use nickel or palladium catalysts that can break apart the cubane structure.
The difficulty in forming and modifying these cubanes means that “1,3- and 1,2-substituted cubanes are largely unknown in medicinal chemistry,” says Mario P. Wiesenfeldt, one of the researchers who led the experimental work on the new methods during his postdoc in MacMillan’s lab. He now runs his own group at the Ruhr University Bochum and the Max Planck Institute for Kohlenforschung.
MacMillan’s team developed solutions to both of these challenges. To produce 1,3-substituted cubanes, the researchers use a light-driven reaction to generate cyclobutadiene, which then reacts with a quinone to generate the cubane. A different reaction sequence can turn the commercially available 1,4-substituted cubane into a 1,2-substituted cubane. Both routes involve four steps, offering much quicker access to the cubanes than before. For now, the new synthetic routes provide 1,2- and 1,3-substituted cubanes in 21% and 35% overall yields, respectively.
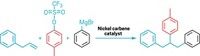
The researchers also found that a copper catalyst offers a way to replace one of the cubane’s ester groups with a wide range of other substituents, including nitrogen heterocycles, alkyl or aryl groups, and trifluoromethane, without disrupting the cubane’s structure. “Any new or optimized methods that functionalize the cubane framework are a welcome addition to the field,” Craig M. Williams of the University of Queensland says in an email. Williams has previously shown that cubanes can be effective benzene bioisosteres (Angew. Chem., Int. Ed. 2016, DOI: 10.1002/anie.201510675).
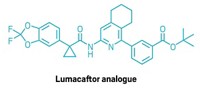
MacMillan’s team used the new methods to make cubane analogs of two drug molecules, including for a cystic fibrosis treatment called lumacaftor. The cuba-lumacaftor maintained high biological activity but had improved metabolic stability and solubility compared to the original drug. This could potentially improve drug absorption in the body.
The new methods will make it easier for chemists to access a broader range of cubanes for medicinal chemistry, says University College London’s Matthew Todd, who has used cubanes to make analogs of a promising antimalarial compound (J. Med. Chem. 2020, DOI: 10.1021/acs.jmedchem.0c00746). “I can imagine that in a couple of years’ time the catalog is going to be full of structures that we can just buy and try, and that’s going to be really helpful,” Todd says. “Or if Dave just wants to mail us some, that’d be awesome.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter