Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceutical Chemicals
NDMA, a contaminant found in multiple drugs, has industry seeking sources and solutions
The presence of the probable carcinogen has prompted drugmakers to issue recall after recall. What risk does it pose, what’s the chemistry behind it, and how are drugmakers going to stop the contamination?
by Leigh Krietsch Boerner
April 20, 2020
| A version of this story appeared in
Volume 98, Issue 15

Credit: Chris Gash
The peak was gigantic. But David Light, a molecular biologist and CEO of the analytical pharmacy Valisure, had seen plenty of big peaks in mass spectra before. This one was at 74 m/z, a mass-to-charge value at which N-nitrosodimethylamine, known as NDMA, might show up. It was 2019, and Valisure had just started checking all its batches of medicines for carcinogens.
In brief
In the past few years, scientists have found the potential carcinogen N-nitrosodimethylamine (NDMA) in multiple pharmaceuticals. While the amounts of the contaminant in the drugs are generally low, some levels have been above the US Food and Drug Administration’s acceptable daily limit, potentially exposing tens of millions of people to a slightly increased risk of cancer. Many of the affected drugs have been recalled, and industry and other labs are scrambling to figure out where the contaminants came from. But there seem to be multiple sources. Read on to learn about those sources and how firms are responding to regulatory agencies’ deadlines to find the origins of the probable carcinogen and get the amounts of NDMA within acceptable levels.
A research associate was using the company’s gas chromatography/mass spectrometry instrument to test one of the first drugs on the firm’s list: an acid reflux baby syrup prescribed to the company cofounder’s daughter. The intensity of the 74 m/z peak was so high that the scientist reran the sample several times to make sure it wasn’t a mistake. Then she took the spectrum to Light. The syrup contained ranitidine, commonly prescribed for heartburn. The scientists would later attribute the intensity of the peak to the conditions under which they analyzed the sample, but the spectrum still indicated that the syrup contained NDMA.
The problem with that is NDMA is a probable carcinogen.
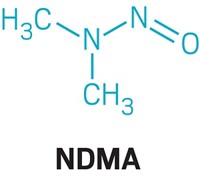
NDMA is an N-nitrosamine, a type of compound that has the generic chemical structure R2N–N=O, a deprotonated amine bonded to a nitroso group. N-nitrosamines are generally formed when a secondary or tertiary amine reacts with a nitrosating agent. The compounds are found at low levels in many foods, such as roasted meats, cheese, and beer, because of cooking and fermentation processes.
The discovery at Valisure wasn’t an isolated incident. In the past few years, private, pharmaceutical, and regulatory agency labs around the world have been finding NDMA and other N-nitrosamine contaminants in various drugs. In 2018, the first discovery was made in a drug containing the active pharmaceutical ingredient valsartan by the drug’s maker, Novartis. Valsartan is an angiotensin II receptor blocker (ARB) used to treat high blood pressure. Since this discovery, NDMA and similar compounds have been found in at least six drugs that are taken by tens of millions of people each year.
Because these N-nitrosamine contaminants are possible carcinogens, regulatory agencies have been deeming the drugs unsafe for people to take and recalling them from shelves. Valsartan was recalled in July 2018, followed that October by irbesartan and in November by losartan, two other ARBs also found to contain NDMA and the related compound N-nitrosodiethylamine (NDEA). In September 2019, the FDA alerted the public to the presence of NDMA in certain lots of ranitidine, available over the counter as Zantac, and manufacturers pulled it from the shelves in the next few months. Nizatidine, another heartburn medication, was recalled by manufacturer Mylan in January 2020. And most recently, the FDA suggested that manufacturers of ranitidine recall all lots and types of these medications. NDMA has also been found in metformin, a diabetes drug taken by over 15.8 million people worldwide. Since May 2020, various companies have recalled more than 170 products containing metformin. The FDA website has a full, searchable list of these products.

The amounts of N-nitrosamines in these drugs may not reach levels that pose a significant risk for patients, but the discovery of the contaminants and the recall of the drugs have caused disruptions for patients across the globe. Meanwhile, drug companies, under the direction of regulatory agencies, are scrambling to figure out how NDMA ended up in such a wide range of medicines and to figure out how to prevent contamination in the future. Experts in the pharmaceutical field point to multiple sources, including side reactions from drug syntheses, the breakdown of unstable drug compounds, and contamination from recycled solvents used in manufacturing.
These contaminants may have been present in our drugs for years, but we didn’t know to look for them, many experts say. In response to this revelation, regulatory agencies are asking companies the question: How can we prevent this from happening again with other potentially harmful contaminants?
What is the NDMA risk?

NDMA is all around us. We’re exposed to it in many ways, but the main sources tend to be tobacco, cured meats such as bacon, fermented foods such as beer and cheese, shampoo and cleansers, and detergents and pesticides. In bacon, for example, NDMA formation occurs when nitrite preservatives react with amines and amino acids in the meat during cooking. NDMA is classified as a group 2A carcinogen, or “probably carcinogenic to humans,” according to the International Agency for Research on Cancer. This means that there isn’t any direct evidence that the compound causes cancer in humans, but it’s likely that it does because it has caused cancer in animals. Both the FDA and Health Canada set an acceptable intake limit for NDMA of 0.096 µg per day on the basis of animal studies. The amount of NDMA that’s been found in pharmaceuticals has varied widely and depended on who did the testing, what manufacturer the drug came from, and what batch of medication was tested.
For the diabetes drug metformin, private labs have detected up to 1.06 µg per dose, more than 10 times the daily recommended limit. For valsartan, one of the recalled lots of the drug contained an amount over 200 times that limit, according to the FDA’s testing. But testing by other agencies couldn’t find NDMA in the majority of valsartan lots. The amounts of NDMA found in nizatidine and ranitidine have also varied widely.
According to Health Canada, the average levels of NDMA found in these pharmaceuticals are not expected to pose a significant increase in cancer risk. “A person taking a drug that contains NDMA at or below the acceptable intake every day for 70 years is not expected to have an increased risk of cancer,” representatives say in an email statement to C&EN. The FDA estimates that if 8,000 people took valsartan at the highest recommended therapeutic dose for 4 years while the drug was contaminated, there would be one additional case of cancer.

The European Medicines Agency (EMA) has also said that there is a low risk of getting cancer from NDMA contamination in medicine. Citing the EMA, Pär Tellner, director of regulatory affairs at the European Federation of Pharmaceutical Industries and Associations (EFPIA), says that no one is suggesting that these medications are toxic. “We’re talking about a small increase in risk of cancers,” he says. Patients should continue to take their blood pressure medications, “because it is more important to control your blood pressure,” he says. “I think you need to put this into some sort of perspective and not panic.”
Still, because people taking drugs with N-nitrosamine impurities over long periods may have even a small increased risk of cancer, the FDA decided to recall some of these medications. The recalls have caused disruptions, especially for doctors and the tens of millions of people in the world who take the drugs to treat chronic illnesses.
The sartan group includes irbesartan, valsartan, and losartan—the last of which is part of the World Health Organization Model List of Essential Medicines. Worldwide in 2017, about 10 million people took losartan, over 2.3 million took irbesartan, and around 1.8 million people took valsartan, according to the Agency for Healthcare Research and Quality. Alternatives to the sartans exist, but switching to a different medication can be problematic, says Erin Michos, director of women’s cardiovascular health and associate professor of medicine at Johns Hopkins University. Many of her patients were on antihypertensive drugs that had been recalled. “These are patients that were stable on their medications,” she says. “Suddenly you’re switching their meds around, and now they have to kind of start over with trying to find the right dose.”
For blood pressure medications specifically, a new drug may make the patient’s blood pressure too high or too low, and finding the right dose of a new drug may take multiple tweaks, Michos says. This means more doctor visits, more monitoring, and more resources such as laboratory tests. And in the case of the sartan recalls, as time went on, the FDA suggested to more manufacturers that they pull more of these drugs off the market. “Sometimes we’d switch to one ARB only to find the one we switched to was recalled as well,” Michos says.
As a result of this hassle and confusion, patients are losing faith in the health-care system, she says. “With this loss of trust between patients and their doctors and trust in their drug supply, it’s even harder to convince patients to take medications they need,” she adds. For instance, several of her patients thought that the danger of getting cancer from NDMA contamination was high, she says, so “they stopped the medicine, not realizing the risk to them is actually quite small.” Michos says she even had patients who stopped taking medications that hadn’t been recalled.

Where is NDMA coming from?
How NDMA ended up in these medications differs from drug to drug. “How to form NDMA is well known,” says Ron Najafi, founder and CEO of Emery Pharma, a contract research organization that has run NDMA tests on multiple pharmaceuticals. By taking what is known about NDMA formation and combining it with knowledge of drug structures and synthesis routes, scientists at regulatory agencies, pharmaceutical companies, private labs, and consulting firms have pieced together possible sources for some of the contamination. The FDA says that the source can be related to the drug’s manufacturing process or even the conditions under which the compounds are stored and packaged. “That explains a little bit why we saw NDMA in varying levels, even in the same drug from the same manufacturer,” says Janet Woodcock, the director of the FDA’s Center for Drug Evaluation and Research. In terms of making NDMA, it’s simple, Valisure’s Light says: “You have two components to NDMA, the N and the DMA,” referring to the nitroso group (N) and the dimethylamine (DMA). To get NDMA formation, you have to get both components near each other. Some of the recalled drugs have both of these pieces built right into their structures, while others do not. This variability makes it difficult to suss out sources and routes for each drug. “It all adds up to a very, very complex picture that can have an impact across a wide range of different drugs,” says Andrew Teasdale, senior principal scientist in impurity management and external advocacy at AstraZeneca, who’s been looking into the issue as it relates to AstraZeneca’s pipeline (Org. Process Res. Dev. 2019, DOI: 10.1021/acs.oprd.9b00535).
For example, with valsartan, there’s neither an N nor a DMA in the final drug, Light says. Ultimately, scientists traced the contamination back to a change in valsartan’s synthesis. The antihypertensive drug contains a tetrazole ring, which is an aromatic five-membered ring with one carbon atom and four nitrogens. For many years, the synthesis for this compound, developed by Novartis, used tributyltin azide to form the tetrazole, with xylene as a solvent. However, in 2014, China’s Zhejiang Huahai Pharmaceutical, which makes valsartan for some companies, filed a patent for an improved method for forming the tetrazole ring. The new route involved swapping out tributyltin azide for sodium azide, which leads to higher yields. The firm also changed the solvent from xylene to dimethylformamide. The new solvent can break down into DMA. So all you would need in order to form NDMA in this situation is some form of a nitrosating agent, Light says. And in this new synthesis, chemists needed a way to get rid of excess sodium azide, so they added sodium nitrite—a possible source of that N.

“As a consequence of changing the chemistry, they introduced the specific risk factors that are needed to ultimately generate N-nitrosamine,” AstraZeneca’s Teasdale says.
Advertisement
Meanwhile, with ranitidine, the NDMA didn’t come from an overlooked side reaction but from the compound itself. This possible breakdown reaction has been known, Light says. “There’s 4 decades of academic research that has been talking about [NDMA] having a carcinogenic potential, specifically from ranitidine,” he says. “[Ranitidine] can even react with itself.”
Ranitidine is a fundamentally unstable drug, Light says. Over time, the molecule goes through a self-degradation process to form NDMA. There’s a lot of speculation about the exact mechanism, but the drug features both amines and a nitrite source, Najafi says. With heat, the degradation happens faster. So if batches of the drug sit in a storage area, NDMA can slowly start forming. Najafi doubts that ranitidine will ever come back to the market, but if it does, it will need to be shipped under temperature-controlled conditions and have a warning label that it’s temperature sensitive. Woodcock says that if manufacturers want to make the drug available again, they would have to demonstrate that the formulation would be stable in any storage conditions that the medicines might encounter.
Ranitidine’s tendency to self-degrade also complicated the tests that Valisure and other firms ran on it. Many of the methods to measure NDMA used by the FDA and other labs involve heating the sample, which means that labs initially saw high levels of the contaminant in their tests, like the huge peaks in Valisure’s baby syrup. As a consequence, the FDA, Valisure, and Emery Pharma all developed low-temperature testing methods for ranitidine.
In the case of metformin, scientists still aren’t sure exactly where the NDMA contamination is coming from. The compound does not have an N, but it does have a DMA, Light says. “It’s a very simple drug and literally a one-step chemical process,” Teasdale says. One of its starting materials is the DMA. “If there’s any sort of nitrosating agent, even in trace levels, it could ultimately lead to that nitrosation of that starting material to form an N-nitrosamine.”
The nitrosating agent could come from any part of the drug manufacturing process, Light says. And drawing a line backward to find the source of contamination is not an easy undertaking.
“You’ve got to look at the whole supply chain right now” to see where the contamination could have originated, says Jim Bruno, director of the consulting firm Chemical and Pharmaceutical Solutions. Maybe the reactors weren’t cleaned properly and NDMA or a nitrosating agent was left behind after synthesizing another drug. To conserve resources, companies sometimes recycle solvents during syntheses, and those solvents could have been previously used in a process in which NDMA formation could occur. “It’s like a domino effect,” Teasdale says.
Some scientists even think contamination may be coming from drug packaging. The FDA says that many pharmaceutical firms have been testing blister packaging and have found low levels of NDMA under certain conditions.
That contaminants might be coming from multiple places makes finding the source that much harder, Teasdale says. “It significantly widens the scope of any investigation because it’s no longer just about the chemistry, and the number of drugs that could be impacted can be much, much higher,” he says.
Bruno also thinks it’s possible that these N-nitrosamine contaminants have been in our drugs for a while. In the past, he says, scientists didn’t have methods of analysis that could detect very small amounts. “But suddenly we’ve got these great methods, so we can see these kinds of things,” leading chemists to detect compounds that they weren’t formerly aware of in pharmaceuticals, Bruno says. He thinks if scientists in the past used today’s instrumentation to look at well-established drugs, it’s possible they would have found unexpected contaminants. “It’s not that the impurities weren’t there; it’s that we just couldn’t see them,” he says.
The potential for the formation of N-nitrosamine impurities in certain drugs was not recognized by regulators and industry until recently, a spokesperson at Health Canada says, which is why this kind of risk assessment wasn’t done during the initial drug approval process. A spokesperson at the FDA echoed this statement: “Before we undertook this analysis, neither regulators nor industry fully understood how the nitrosamines could form during the manufacturing process.”
But regulatory agencies are looking for them now. “It’s very clear that if [NDMA contamination] appears in a number of products, then you need to take a step back and really make sure that you do not have this problem in more products,” Tellner of the EFPIA says.

Sources: US Food and Drug Administration, 2018–20; Toxicol. Res. 2015, DOI: 10.5487/TR.2015.31.3.279; Scanlan, Richard A. “Nitrosamines.” In Encyclopedia of Food Sciences and Nutrition, 2nd ed., edited by Benjamin Caballero. Academic Press, 2003. a The amount of NDMA is for 12 g of bacon (one slice). b The amount of NDMA is for 42.52 g of cheese.
What is being done?
Now that regulatory agencies are aware this problem exists, they’re pushing companies to act. Both the EMA and Health Canada have released guidelines directing the pharmaceutical industry to perform risk evaluations of all drugs and review manufacturing processes to find any risk of creating N-nitrosamine impurities. This involves all the major pharmaceutical companies, Teasdale says. AstraZeneca is evaluating its entire drug portfolio. “This is looking at all of the different risk factors, primarily focused on the chemistry but also looking at things like packaging and any contribution coming from the formulated product,” Teasdale adds.
The EMA released its guidelines in September 2019 and gave companies 3 years to complete all the mandated steps. The first step is an initial assessment of high-risk products, originally due on March 26. This date has since been pushed back to Oct. 1 because of the COVID-19 pandemic. Health Canada released similar guidelines in December 2019 and gave industry 2 years to get nitrosamine impurities to below acceptable limits in all drugs.
The FDA has yet to put out any guidance on the matter but is planning to soon, according to Woodcock.
What companies will do after this initial assessment depends on what they find and where they find it, Tellner says. If NDMA is found in a drug, it may or may not be pulled from the market. “It depends on if alternative products exist on the market that you could switch to until this has been corrected,” he says. “This kind of assessment needs to be made by [regulatory agencies] and the company.”
Either way, Bruno, a consultant, doubts that the results of these internal assessments will be made public.
And what about new drugs in development? For drugs coming onto the market, an NDMA check is now likely to be part of the testing process, Bruno says. If companies see a possible NDMA-forming side reaction, he says, they can redesign the process to avoid it.
“We obviously need to have increased vigilance in these areas,” Valisure’s Light says. He proposes developing a system that can score drug safety in terms of testing for impurities and how well a manufacturer complies with regulatory oversight. The FDA has talked about the idea of quality scores for drug products and drug manufacturers multiple times, he says. In December 2019, Woodcock released a white paper about holding pharmaceutical makers to a quality management maturity standard to ensure that the US drug supply remains safe. Right now, the FDA just issues warnings to manufacturers if they don’t meet standards, Woodcock says. “We are very interested in putting out some kind of incentive program where we can award recognition to companies that have very high, outstanding quality.”
This type of quality score could be powerful, Light says, because there’s little transparency of drug quality. “You have that in almost every other business when you buy something.” For instance, if you buy a car, you can check the vehicle’s history report from a company like Carfax, he says, “but with drugs, you just get an orange bottle with pills in it.”
And more transparency about monitoring for impurities in drugs is needed. It’s possible that we may find another carcinogen contaminating drugs, Bruno says. “My personal opinion is we’re going to see this again,” because it’s something the pharmaceutical industry has been dealing with knowingly and unknowingly for years. “I’d put money on it.”
UPDATE
This story was updated on May 29, 2020, to add that the FDA recommended the recall of some metformin products on May 28, 2020.
This story was updated on July 13, 2020, to add that two companies, Granules Pharmaceuticals and Lupin Pharmaceuticals, recalled metformin products in early July 2020.
This story was updated on Oct. 12, 2020, to say that multiple companies have now recalled metformin products since May 2020. The full list of recalled metformin medications can be found here at the FDA website.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter