Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Process Chemistry
A side reaction may have led to impurities found in valsartan heart drugs
Multiple manufacturers have been involved in drug recalls over the past eight months
by Tien Nguyen
February 19, 2019

Over the past eight months, more than a dozen pharmaceutical distributors around the world have voluntarily recalled hundreds of batches of generic versions of the drug valsartan and related products losartan and irbesartan, which are commonly prescribed to treat high blood pressure and heart failure. The most recent recall occurred earlier this month. According to the Agency for Healthcare Research and Quality, about 1.6 million people purchased valsartan in the US in 2016.
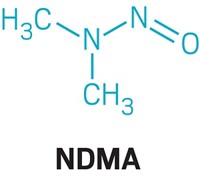
The recalled products contained active pharmaceutical ingredients (APIs) with trace amounts of either N-nitrosodimethylamine (NDMA) or N-nitrosodiethylamine (NDEA), which are both probable human carcinogens. Testing by companies and the US Food and Drug Administration has revealed that the impurities came from multiple API manufacturers, facilities that synthesize the biologically relevant compounds—valsartan and related sartan molecules—and then sell them to companies that make the final tablets that reach consumers. Based on information about how one of these API manufacturers produces the compound and reports from that company, experts think that these impurities were made through a side reaction of one step in the compound’s synthesis.

The four API manufacturers that produced the impure sartans are Hetero Labs, Mylan Laboratories, and Aurobindo Pharma, based in India, and Zhejiang Huahai Pharmaceutical (ZHP) in China. ZHP was the first to disclose the presence of the NDMA impurity after receiving a complaint from a customer in June 2018 about an unidentified impurity in valsartan’s APIs which the company received from ZHP. By July 2018, Prinston Pharmaceuticals and Teva Pharmaceuticals had recalled valsartan products that contained contaminated APIs supplied by ZHP. More recalls involving ZHP and the other manufacturers followed. For example, the following month Camber Pharmaceuticals recalled multiple valsartan batches with APIs from Hetero Labs and in October ScieGen voluntarily recalled irbesartan products containing APIs from Aurobindo. Torrent Pharmaceuticals began recalling batches of losartan with API from Hetero Labs in December 2018 and expanded their recall in early 2019. Earlier this month, Pfizer Japan recalled three-quarters of a million drug tablets containing valsartan APIs from Mylan Laboratories.
FDA testing found that, in some cases, the impurity concentrations in valsartan exceeded the agency’s acceptable intake levels for people—0.3 ppm for NDMA and 0.08 ppm for NDEA. NDMA concentration levels in the APIs ranged from 0.156 ppm to 63.09 ppm. The risk of these doses of the impurities is relatively low. The FDA’s scientists estimate that if 8,000 people took the highest valsartan dose (320 mg) from the recalled batches daily for four years, there may be one additional case of cancer over the lifetimes of those people.
According to an internal investigation of the impurity by ZHP, NDMA formed during their manufacturing process. ZHP and the other API manufacturers make their generic valsartan through methods that differ from how Novartis, which developed the drug, originally synthesized it. ZHP implemented their updated process in 2012 after it was approved by relevant regulatory agencies including the FDA. Based on ZHP’s records, “some levels of the impurity may have been in the valsartan-containing products for as long as four years,” according to an FDA statement.
Philippe Andre, a pharmacist and auditor at the China-based company Qualandre, thinks the impurity was probably produced in a key tetrazole-forming step in the synthesis. Andre has inspected ZHP’s facilities for good manufacturing practices (GMP) compliance for drug-product clients, but he does not test substances or review synthetic routes. His understanding of how ZHP makes the drug is based on a 2014 Chinese patent assigned to ZHP for the improved method for forming the tetrazole ring of valsartan. For this key step, ZHP uses sodium azide, which leads to higher yields compared with the original patented route from Novartis that uses tributyltin azide.

This reagent switch requires chemists to get rid of excess sodium azide by adding sodium nitrite, which under acidic conditions can form nitrous acid. Andre says nitrous acid likely reacted with small amounts of dimethylamine to produce NDMA. Dimethylamine is a degradation product of the solvent used in the reaction, dimethylformamide.
A Novartis spokesperson told C&EN that, based on company chemists’ understanding of ZHP’s process and public information released by agencies like FDA, this sodium azide-based side reaction is likely how NDMA formed during the synthesis. Pharmaceutical industry insider, Derek Lowe, suggested the same synthetic pathway in a blog post on the contamination issue. Information posted on ZHP’s website in August 2018 also supports this explanation: “The impurity was generated in the side reaction between a trace-amount of degradate of a solvent and the reagent in the downstream step.” As of last week, however, the page no longer appears on ZHP’s site. ZHP did not respond to C&EN’s requests for comment on the origin of the impurity by press time.

According to the FDA, Hetero Labs uses a process similar to ZHP’s to manufacture valsartan but the levels of NDMA detected in their products have been generally lower than those found in the ZHP products. In a statement to C&EN, FDA did not confirm the route to the impurity but noted that an investigation is ongoing. They added that “the reuse of materials, such as solvents, may cause the presence of an impurity in processes which otherwise do not appear to be at risk for forming these impurities.” The FDA declined to explain which materials may have been reused and which impurities may have formed as a result.
The synthetic origins of the NDEA impurity, however, is still unclear. In his blog post, Lowe suggested that it could have formed through a similar chemical route involving diethylformamide as a solvent instead of dimethylformamide. C&EN has not been able to confirm that any of the API manufacturers used diethylformamide in their sartan production.
Andre says he finds it strange that the introduction of sodium nitrite into the reaction mix for the tetrazole step didn’t raise red flags for chemists at the companies or for regulators who assessed the new process. In his opinion, chemists evaluating the reaction conditions should have considered the possibility of this side reaction. He suggests that more awareness from manufacturers and better training for regulatory assessors could have prevented the contamination, adding that the incident should be a “wake-up call” to regulators.
Advertisement
Over the past decade, the FDA has been making the analytical requirements for APIs much more stringent, says Ed Price, chief executive officer of the API and drug-substance manufacturer SEQENS North America. Price says that although the FDA missed this possible side reaction when ZHP applied for approval in 2012, he doubts they’d miss it today. “They’ve raised the bar so much since 2010, there’s not much more they can do.”
The FDA tells C&EN that before they began their investigation of the valsartan recalls, “neither regulators nor industry fully understood how NDMA or NDEA could form during this particular manufacturing process.”
The agency says that the impurities were hard to detect using the analytical methods provided by the manufacturers, especially given the compounds’ low concentrations. Andre explains that the problem is “you’re not going to see it if you don’t know that it’s going to be there.”
To help industry and regulatory agencies keep track of the impurities, the FDA has published three gas chromatography/mass spectrometry methods to detect NDMA and NDEA.
On the now-deleted webpage from August 2018, ZHP announced it was developing a new manufacturing process to avoid the generation of NDMA. Currently, all API made at ZHP’s Chuannan facility is barred from entering the US following an import alert issued by the FDA in September 2018.
CORRECTION:
This story was updated on Feb. 20, 2019, to correct the caption for the possible route to the NDMA impurity. The nitrite added to the reaction is the species that forms nitrous acid.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter