Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Vaccines
On the hunt for alternatives to shark squalene for vaccines
Advocates want vaccine makers to make adjuvants from plant sources instead
by Melody M. Bomgardner
December 6, 2020
| A version of this story appeared in
Volume 98, Issue 47

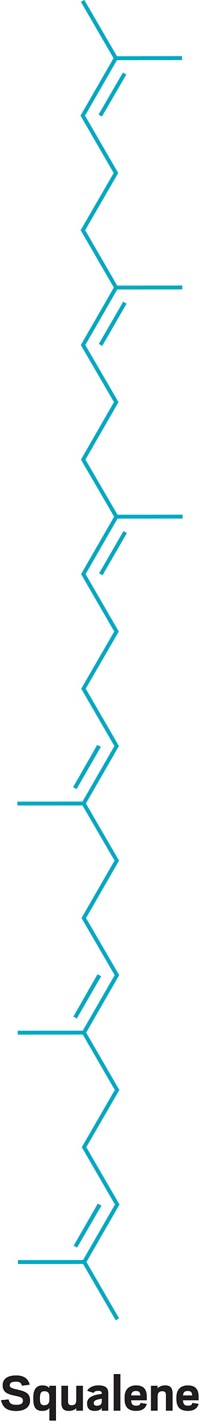
Sharks use it to stay buoyant. Cosmetics makers use it to soften skin. But in the time of COVID-19, squalene’s use in vaccine formulations is what’s bringing attention—and some controversy—to this natural lipid.
Squalene plays a powerful role in some of the additives, called adjuvants, that boost the body’s immune response to a vaccine’s active ingredient. And the most concentrated source of high-purity squalene is the livers of sharks, particularly in shark species that live in deep water.
The global trade in pharmaceutical-grade squalene, as for shark parts like fins, relies on sharks caught either on purpose or accidentally with other fish. Most squalene is used to make squalane for cosmetics; demand from the pharmaceutical industry is not known but is thought to be much smaller.
Still, marine conservationists, suppliers of squalene from nonshark sources, and some scientists say vaccine adjuvant makers including GlaxoSmithKline, Merck KGaA, and Novartis should take steps to move to nonshark sources of squalene. “If you care about the ocean, you have to care about sharks,” says Stefanie Brendl, executive director of the advocacy group Shark Allies.
Currently, there are two shark-free sources of squalene. The biotech firm Amyris has developed a semisynthetic pathway that starts with sugar fermentation. Squalene can also be extracted and purified from the leftovers of olive-oil refining. Both methods have made inroads into the cosmetics industry, but neither has entered the more highly regulated pharmaceutical supply chain.
And companies could tap other sources as well. As far as scientists can tell, pretty much all plants and animals produce squalene. It is a precursor to sterols, such as cholesterol, and steroids. It’s even in sebum, the oily stuff that makes your nose shiny.
Today, adjuvants containing squalene from sharks are used in common flu vaccines containing viral proteins, parts of the virus, or a disabled virus. So far, five of the dozens of COVID-19 vaccines in development also use squalene, though messenger RNA vaccines by Pfizer and Moderna do not, according to the World Health Organization.
At least one company, GSK, expects squalene-containing COVID-19 vaccines will be approved and distributed. It is gearing up to supply adjuvants for 1 billion doses in line with its partnerships with the vaccine developers Sanofi, Clover Biopharmaceuticals, Medicago, and Innovax. And while GSK is looking into nonshark sources, they won’t be ready in time for the COVID-19 crisis, the company says.
Wildlife advocates are dismayed that potentially billions of COVID-19 vaccine doses will contain squalene extracted from the livers of sharks, some of them endangered species, that are harvested from the ocean.
“No one is saying people are going out to catch hundreds of thousands of sharks just for this use, but as it becomes more normalized and as you have global demand, with billions of people needing something, it gets harder and harder to move away from this source,” Brendl says. “What we are asking is to start testing alternatives so that eventually we don’t have to keep using this animal product.”
People, and not just sharks, would benefit if the drug industry were to stop relying on shark squalene, contends John Melo, the CEO of Amyris. Squalene is currently used sparingly, he says, and often saved for doses administered to older people or children—people with weaker immune responses. Investments in biomanufacturing could mean more vaccines would benefit from squalene-containing adjuvants.
“We believe squalene is not used more because of access and cost,” Melo says.
If squalene were plentiful, Melo adds, vaccine makers could use its immune-boosting quality to decrease the quantity of the vaccine active ingredient, known as the antigen, in each dose. That antigen-sparing function would make it easier to quickly scale vaccine production.

Moreover, vaccines with less protein or virus are less likely to cause major side effects. Lastly, when squalene adjuvants are added, they help stabilize the protein or virus so the vaccine can be stored and shipped without freezing.
But how did shark liver oil end up in vaccines in the first place? Until the 1980s, vaccine adjuvants contained mineral oil, which is easy to get but not biocompatible. Christopher Fox, vice president of formulations at the nonprofit Infectious Disease Research Institute (IDRI), says scientists evaluated a long list of natural lipids to find one that triggered the immune system but was easier to tolerate. And squalene just happened to fit the bill.
Vaccine scientists have learned that for squalene to do its immune-boosting work, it has to be delivered in a specific form—as nanodroplets emulsified by a phospholipid or nonionic surfactant, Fox says. GSK’s AS03 adjuvant, for example, contains squalene, α-tocopherol, and polysorbate.
When delivered as part of a vaccine injection, the oily droplets increase cellular uptake of the vaccine antigen and help recruit immune cells to the site of immunization. Immune cells also carry the antigen into the lymphatic system, which signals the immune system to ramp up.
How squalene triggers these responses is still a bit of a mystery. “There’s a lot we don’t know. We haven’t identified a specific receptor that squalene binds to,” Fox says. “The inflammasome and other innate immune pathways may play a role.”
Fox is working on federally funded pandemic-flu preparedness programs to devise sustainable raw materials for adjuvants and establish large-scale adjuvant manufacturing. A side benefit of that work, he says, is the ability to create analogs of squalene to help researchers understand why, and how, it contributes to immune activity.
Separately, IDRI has partnered with Amyris on two vaccines—one for the Zika virus and one for COVID-19—that contain squalene made by the biotech firm. Although they have yet to enter clinical trials, approval by the US Food and Drug Administration would mark the first use of semisynthetic squalene in a commercial vaccine.
IDRI’s COVID-19 vaccine is based on replicating RNA rather than mRNA or a protein or other virus material. Because a typical innate immune response to viral RNA is to immediately take it apart, the role of squalene in an RNA vaccine is different than in traditional vaccine adjuvants, Fox explains. Squalene-containing lipid nanoparticles act as carriers to deliver RNA, attached to their surfaces, into cells. In that system, squalene does not juice the immune response as much as in traditional adjuvants.
Pfizer and Moderna use different lipid nanoparticles as the carriers for their mRNA vaccines.
Melo says safety and nonclinical efficacy trials have been completed on Amyris’s semisynthetic squalene. The company is in the process of scaling up and says it would be able to meet the need for 4 billion vaccine doses over the next few months.
“We have about five pharma companies now engaged, including some of the world’s largest,” Melo reports. “We’re sampling; they’re testing. We’ll have our first supply by the end of the year.” He declines to identify what vaccines are being targeted.
To make squalene for vaccines, Amyris is tweaking its scaled-up process for squalane, the hydrogenated form of squalene used in cosmetics. The company feeds cane sugar to a microbe modified to produce farnesene, a sesquiterpene. From there, Amyris uses chemical synthesis to produce squalene or squalane. It supplies squalane to cosmetics makers and uses it in its own Biossance line of skin-care products.
The cosmetics-first route is also how the French natural ingredients firm Sophim got into the squalene business. Its biggest market has always been cosmetics, but since the 2009 H1N1 swine flu pandemic, it has sold shark-derived squalene to the pharmaceutical industry, Managing Director Alexis Margnat tells C&EN in an email.
Over the last 15 years, the European cosmetics industry has abandoned shark squalane. So Sophim built capacity to make plant-derived squalane by extracting squalene from a distillate left over from olive-oil manufacturing and hydrogenating it. The distillate contains 3–10% squalene. Margnat estimates the olive-oil squalane market is about 1,000 metric tons per year and says Sophim is the largest supplier from its factories in France and Spain.
Now, Sophim is working to purify olive-oil squalene to meet pharmaceutical standards. That requires removing small amounts of sterols, tocopherols, and paraffins, which is not easy to do, Margnat says.
Not all adjuvants that assist vaccines contain squalene. The list of COVID-19 vaccine candidates includes one from Sinovac that uses aluminum hydroxide. Sinovac and Clover have also tapped Dynavax Technologies for its toll-like receptor 9 agonist, CpG 1018, already used as an adjuvant in Dynavax’s hepatitis B vaccine.
GSK says growth in demand for squalene adjuvants will not result in harm to sharks. The company says its supplier, which it won’t name, does not use sharks listed as endangered species under CITES, the Convention on International Trade in Endangered Species of Wild Fauna and Flora.
But Brendl from Shark Allies says the global trade in shark parts is not transparent, and it may not be possible to prove that squalene came from a healthy shark population or species. “Every product that uses shark products contributes to the taking of 70–100 million sharks per year,” she says.
Correction
This story was updated on Dec. 8, 2020, to correct the sugar source for Amyris’s production of farnesene. It is sugarcane, not corn.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter