Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Chemistry In Pictures
Chemistry in Pictures: Soap star
by Alexandra A. Taylor
September 24, 2020

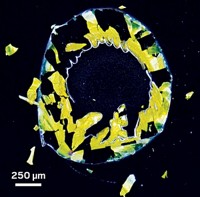
This photo shows “the monolayer formed by tiny soap bubbles that are saving the world during the ongoing COVID-19 pandemic,” says Ankita Naik, a PhD candidate at the University of Minnesota Twin Cities. Naik formed the monolayer by pouring a few drops of soap foam onto a petri dish filled with a soap solution. She uses images like this one to compare the bubble size distribution—an indicator of soap foam stability and lather formation—for various soap foams. Monitoring this information can give researchers clues about how a given soap film will drain and coalesce under various conditions. Naik says her lab has developed a new class of biorenewable surfactants, known as oleo-furan surfactants, that are 100 times as stable as conventional, petrochemical-based surfactants in hard water, characterized by high levels of calcium and magnesium minerals. Her research focuses on the kinetics and mechanisms of key reactions involved in synthesizing these surfactants, which she hopes will explain their improved hard-water tolerance.
Submitted by Ankita Naik
Do science. Take pictures. Win money. Enter our photo contest here.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter