Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Record-breaking molecular magnet
Dilanthanide complexes could pave the way for a new breed of powerful permanent magnets
by Mark Peplow, special to C&EN
January 13, 2022
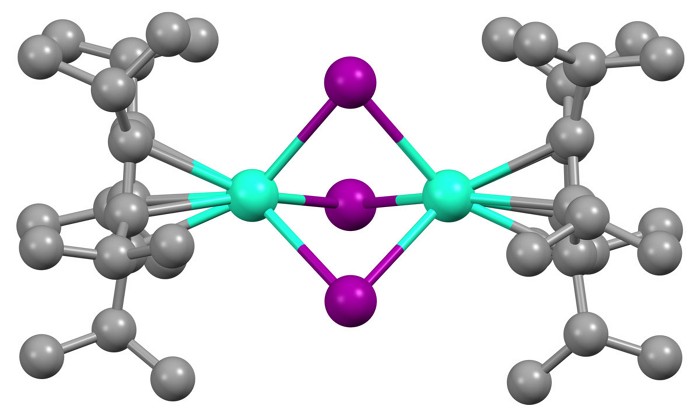
By coupling together a pair of lanthanide ions within the same compound, researchers have created what they believe are the most magnetic molecules ever made (Science 2022, DOI: 10.1126/science.abl5470).
“By all the traditional metrics of single-molecule magnets, they’re the best,” says Nicholas Chilton of the new molecules. Chilton, who’s based at the University of Manchester, collaborated on the work with Jeffrey Long at the University of California, Berkeley, and Benjamin Harvey at the US Naval Air Warfare Center Weapons Division. Although the molecules’ magnetism only reveals itself at low temperatures, Chilton hopes that these dilanthanide complexes might pave the way for new types of powerful yet lightweight permanent magnets.
Roberta Sessoli of the University of Florence, a pioneer of single-molecule magnets who was not involved in the work, says “this really is a very, very important piece of work. This is something that is going to remain as a milestone.”
Lanthanides, such as neodymium and samarium, partner with transition metals in the strongest rare-earth magnets, which are used in some electric vehicle motors and wind turbines. In rare-earth magnets, metal-metal bonds help to align unpaired electrons in the lanthanides and their transition metal partners, boosting the overall magnetism. Coupling two lanthanides in this way should lead to even greater magnetism, but it has proved difficult to forge bonds between them.
The new complexes overcome that hurdle. They contain a pair of lanthanide ions—terbium or dysprosium, for example—bridged by three iodide anions and capped by bulky aromatic ligands. A single, shared electron sits in a bonding orbital between the two ions, and this helps to align all the unpaired electrons on both ions. Although lanthanides have previously bonded together inside fullerenes, “as far as I’m aware, these are the first conventional molecular lanthanide-lanthanide bonds,” Chilton says.
The researchers used several methods to assess the molecules’ magnetic properties. One test involved using an external magnetic field to flip the molecule’s magnetic orientation. The stronger the molecule’s magnetism, the larger the field required to flip it, Chilton says.
When the terbium and dysprosium complexes were chilled below 50K and 60 K, respectively, this so-called coercive magnetic field was absolutely enormous—far larger than for any other molecule or commercial permanent magnet. Both complexes easily withstood the hefty 14 T field of a conventional magnetometer. Further experiments on the terbium complex suggest its coercivity tops 25 T. In comparison, the previous record for a single-molecule magnet was 7.9 T at 10 K. The dysprosium complex also remained strongly magnetic at a relatively balmy 80 K, matching the highest recorded operating temperature for a single-molecule magnet, and warmer than the 77 K chill of liquid nitrogen.
“They beat all the records in this paper,” says Rodolphe Clérac of the University of Bordeaux, who works on molecular magnets. “It’s very exciting, every stage of this work is a tour de force.”
“It’s a spectacular result,” agrees William J. Evans of the University of California, Irvine, who collaborates with Long on lanthanide chemistry but was not involved in this work. “What I like about it is it’s such a simple system.” The synthesis of the new molecules builds on work by Evans and colleagues, who had developed complexes containing lanthanide ions in an unusual +2 oxidation state. “I think this is going to be a new paradigm for making molecules like this,” Evans says.
Chilton suggests that future lanthanide complexes might be used in nanoscale magnetic devices, or assembled into larger magnets. “I think there is actually scope for making a macro-scale magnetic material from this kind of thing,” Chilton suggests.
Meanwhile, Clérac thinks the new compounds could help to develop magnetic molecules for data storage applications, in which the complexes can be switched between two stable magnetic states that represent the ones and zeros of binary logic. “I think this is going to be a key paper in the field for the next 20 years,” he says. “It’s going to be cited by everybody.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter