Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Astrochemistry
Electrochemistry could explain Mars’s organics
Brines and metal-rich minerals could reduce CO2 to make alcohols and more
by Sam Lemonick
November 12, 2018
| A version of this story appeared in
Volume 96, Issue 45

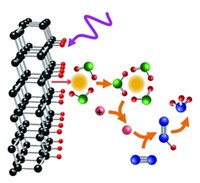
Scientists have identified aliphatic and aromatic organic molecules on the surface of Mars and in martian meteorites. The source of these molecules is unknown, but some scientists suggest they formed during meteorite impacts or through geological processes. Now one team proposes a novel explanation: As salty liquids flowed through microscopic fissures in metal-rich minerals on the red planet, electrochemical reduction of carbon dioxide (ERC) generated the small organic molecules (Sci. Adv. 2018, DOI: 10.1126/sciadv.aat5118). Chemists are intrigued by the mechanism, but some say they want to see more evidence that the reactions are plausible.
Andrew Steele of the Carnegie Institution for Science had been studying martian organics when he noticed signs of corrosion in meteorites in which the organic molecules had been found. The corrosion made him wonder if an electrochemical process could have created the molecules. That led him to ERC, a process that chemists have used in which an electrochemical potential drives reduction of CO2 to yield CO and simple organics.
Steele and his colleagues propose that neighboring micrometer-thick layers of iron-rich and iron-poor minerals could act as electrodes in a sort of galvanic cell. Brine moving through cracks between the two would serve as the electrolyte. At low pH, Steele says, electrochemical reactions could reduce CO2 in the brine to make organic molecules like methane and formate.
“This inspiring study shows how materials complexity can translate into very interesting localized reaction conditions,” electrochemist Frank Marken of the University of Bath says. But he and others are skeptical that the proposed system has enough energy to reduce CO2 and suggest another source, such as light, might be needed.
Steele says he’s working to replicate the system in a lab. But he explains that electrical potentials can increase dramatically in nanoscale galvanic cells, potentially providing the energy needed.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter