Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
Actinide acts as electron donor for first time
Thorium donates electrons to aluminum in first-of-its-kind complex
by Sam Lemonick
May 2, 2018
| A version of this story appeared in
Volume 96, Issue 19
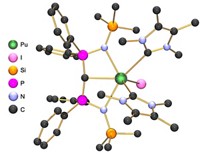
A new thorium-aluminum complex is the first in which an actinide element donates electrons when bonding with a metal (Chem. Sci. 2018. DOI: 10.1039/c8sc01260a).
The complex, synthesized by John Arnold of the University of California, Berkeley, and colleagues, is already unusual because its thorium atom is in a +3 oxidation state. Chemists have made fewer than 10 Th(III) complexes to date. The energy and symmetry of its highest orbitals mean the element is almost always in a +4 oxidation state. Arnold’s group was trying to better understand Th(III)’s electronic structure when they stumbled on some unusual bonding behavior.
To stabilize this reduced form of thorium, the chemists coordinated it to a bulky alanate ligand. Density functional theory (DFT) calculations predicted delocalization of the electron in thorium’s highest occupied orbital onto the aluminum atom. Electronic paramagnetic resonance spectroscopy showed the two atoms were sharing electrons in a covalent interaction. Further DFT studies indicated that thorium was indeed donating an electron to the aluminum.
Stosh Kozimor, an actinide chemist at Los Alamos National Laboratory, thinks this compound could be the first of many like it. “I predict that Arnold and coworkers have uncovered a bonding interaction that may have substantial implications on reactivity that transcends hundreds of compounds containing [actinides] linked to main group Lewis acids,” he says.
Arnold agrees. If chemists want to make more kinds of metal-actinide bonds, it helps if the actinide can be an electron donor and not just an acceptor, he says.
Arnold says the research, which was funded by the U.S. Department of Energy, could be most useful in making alloys of the heavier actinides, like plutonium. In nuclear weapons, these elements are stabilized by metals like gallium. Making and understanding stable alloys is vital for nuclear technology, he says.
CORRECTION: This story was updated on May 3, 2018, to correct the conclusions of the density functional theory calculations of the complex. The calculations indicated that thorium donated one electron to aluminum, not multiple electrons.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter