Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
Chemists predict ghostly molecule
Electromagnetic fields could create molecule-like electron density around single atom, according to computer simulations
by Sam Lemonick
September 19, 2018
| A version of this story appeared in
Volume 96, Issue 38
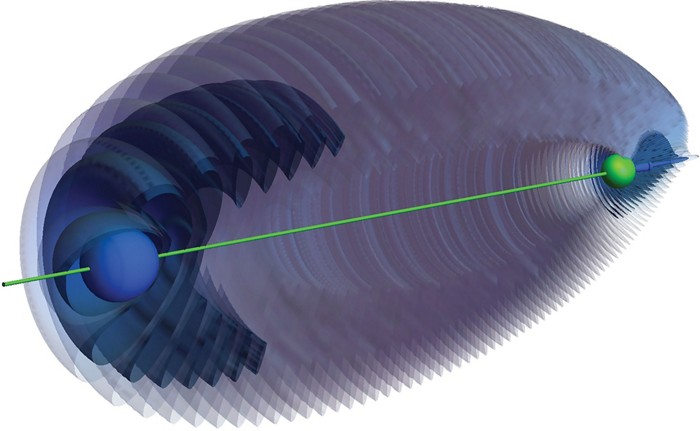
You might want to leave the lights on tonight; scientists say ghosts can exist. Theorists predict conditions to create a so-called ghost molecule—a single atom with electron density that resembles that of unusual two-atom molecules (Phys. Rev. Lett. 2018, DOI: 10.1103/PhysRevLett.121.113203).
Besides expanding chemists’ understanding of chemical bonding, these oddities could serve as components of quantum computers, if researchers can find a way to make them.
These ghosts resemble trilobite molecules, which Chris H. Greene of Purdue University and colleagues predicted in 2000. The trilobites consist of a Rydberg atom—one in which electrons are in very high energy states—and a second atom whose presence causes the Rydberg atom’s energy levels to mix together to create an unusual pattern of electron density that looks like a fossilized trilobite. Researchers at the University of Oklahoma made a trilobite molecule from cesium atoms in 2015.
Greene’s group is now predicting that the same effect can be achieved using an excited hydrogen atom and a sequence of electric and magnetic field pulses to perturb its energy levels. Because there is no second, ground-state atom, the team calls the effect a “ghost bond” or “ghost molecule.”
Through computational simulations, they predict the resulting trilobite-like electron density would last several milliseconds at 10 K. Like with non-ghost trilobite molecules, these densities would be large—as much as 2,500 angstroms long—and have permanent dipole moments on the order of 1,000 Debye.
Harvard University theoretical physicist Hossein Sadeghpour, who first predicted the existence of trilobite molecules with Greene, says “it will take awhile” to make the ghost version, but he thinks it is possible. He and Greene agree detecting them may be as hard as making them because it is difficult to image these high energy states. Greene proposes using electron momentum spectroscopy or X-ray diffraction spectroscopy, but he thinks it could be a stretch even for those techniques.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter