Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
Electrochemistry of single molecules under the microscope
AFM explores relationships between charge, bonding, and structure
by Mark Peplow, special to C&EN
July 12, 2019
| A version of this story appeared in
Volume 97, Issue 28

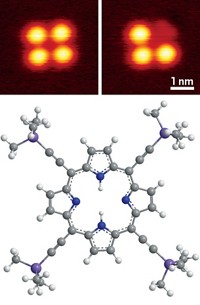
An atomic force microscope (AFM) has revealed how the structure and bonding patterns of organic molecules change as they take on electrical charges (Science 2019, DOI: 10.1126/science.aax5895). The technique could be used to study photosynthesis, organic photovoltaic devices, and molecular electronics. “How structure changes with charge is at the very heart of these processes; it’s fundamental,” says Leo Gross of IBM Research–Zurich. The researchers used an AFM to study four molecules adsorbed on an insulating sodium chloride film. By changing the voltage between probe and sample, the AFM could remove or add electrons from single molecules and then map the positions of the atoms. Adding an electron to azobenzene, for example, disrupted its conjugated system and caused its phenyl rings to twist out of their usual coplanar arrangement. In the diradical dianion pentacene, AFM showed that carbon atoms in the second and fourth rings hosted the unpaired electrons. And for porphine, the parent compound of porphyrins such as chlorophyll and heme, the technique tracked how bonding conjugation changed between the neutral, aromatic system and its antiaromatic dianion. The team hopes to use this method to perform single-molecule synthesis, moving atoms and charging them to make or break individual chemical bonds.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter