Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
Errors in C&EN graphic reveal widespread misconceptions about slime chemistry
Multiple sources, including journal articles and chemical catalogs, get the borate bonding and reactivity wrong
by Mitch Jacoby
July 3, 2018
| A version of this story appeared in
Volume 96, Issue 28

When Chemical & Engineering News hears from readers that it has published an incorrect chemical structure in an article, writers and editors check the story’s source material to confirm the error and then run a correction pointing out the mistake. But what should C&EN do when the source material, including a number of reputable sources, is also incorrect?
This certainly doesn’t happen often. But it’s a situation that we found ourselves in about three weeks ago, when we published a graphic to accompany a story about the popularity of slime, the gooey, puttylike material often made in classroom and home science demonstrations. The graphic described the chemistry of slime and showed two key structures—one of borax, an ingredient used to make slime, and one of the cross-links that hold slime together. A few eagle-eyed readers messaged C&EN to point out errors in both of these.
A little scientific sleuthing quickly showed us that we weren’t alone in making the mistakes. Many sources, including databases, journal articles, university websites, and chemical catalogs, also presented incorrect structures for borax and these cross-links. We won’t name any of those sources, but suffice it to say, a Google search for “slime” + “hydrogen bonds,” which we now know are not the main type of cross-link found in slime, gets more than 30,000 hits. So we interviewed some experts to get to the bottom of these inaccuracies and why they’re rampant.
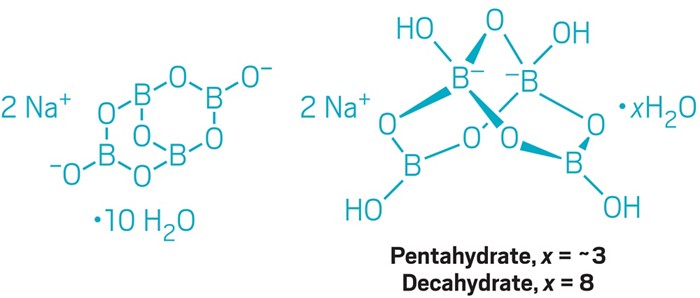
Let’s start with borax. Also known as sodium tetraborate decahydrate, this boron compound is used on an industrial scale to make laundry detergents and other products. The decahydrate is closely related to a pentahydrate compound, which is produced on an even larger scale and used in fertilizers and glassmaking.
“I’m not sure why so many people get the structures of these important industrial borates wrong. Good crystal structures have been available for them for a long time,” says David M. Schubert, a research chemist who worked at U.S. Borax for nearly 30 years before taking a position teaching organic chemistry at Metropolitan State University of Denver.
For borax decahydrate, the structure dates back to a 1956 study published in Mineralogical Journal. Later papers refine the structure but agree with the original study’s main points. They show that the tetraborate anion, [B4O5(OH)4]2–, contains two boron atoms with tetrahedral bonding geometry and two others with trigonal geometry. Borax pentahydrate exhibits the same bonding motif.
The incorrect structure that we published can be found in chemical catalogs and shows trigonal bonding at all four boron atoms. Schubert proposes that perhaps the structure errors stem from organic chemists trying to make these compounds fit organic bonding patterns, which doesn’t work. He says that even boron chemists are often unfamiliar with the details of boron-oxygen compounds because they typically focus on organoboron or boron hydride chemistry.
Brendan Yonke agrees. He’s a senior research chemist at Rio Tinto Borates, which was created by Rio Tinto Group’s acquisition of U.S. Borax. “Boron-oxygen chemistry isn’t widely known. It’s kind of a long-lost art that isn’t taught in school anymore.”
Advertisement
Bangor University’s Michael A. Beckett feels that industry is partly to blame for these misconceptions. Beckett is an inorganic chemist who specializes in borate and silicate compounds. He points out that manufacturers were using borates commercially long before their structures were known, and these large-scale users often named the compounds as hydrated oxides strictly on the basis of stoichiometry.
As a result, borax is commonly formulated as Na2B4O7·10H2O and referred to as a decahydrate. Its true formula, Beckett stresses, is actually Na2[B4O5(OH)4]·8H2O—the octahydrate of the sodium-coordinated tetraborate anion. Both formulas specify the same number of atoms but indicate different chemical structures.
The second incorrect chemical structure in C&EN’s slime graphic depicts the cross-linking that results when poly(vinyl alcohol) (PVA) and borax mix. That reaction converts the low-viscosity solutions of starting materials to slow-flowing slime.
In our depiction, tetrahydroxyborate ions serve as hydrogen-bonding cross-links between PVA chains. As with the borax structure error, hydrogen-bonded PVA shows up all over the internet, as well as in journal articles and books. Yet borate specialists say it’s incorrect and that borate ester groups do the cross-linking. This issue may be less clear cut than that of the borax structure.
Mahesh K. Mahanthappa, a specialist in soft materials synthesis at the University of Minnesota, Twin Cities, explains that slime making is a dehydration reaction that forms B–O ester bonds to the PVA chains. The ester groups do the bulk of the cross-linking and give the product its slimy character. But as Schubert points out, the B–OH groups in this system will form some hydrogen bonds to oxygen atoms. They’re just not the predominant type of cross-link in slime.

Experts interviewed by C&EN suggest that one reason hydrogen bonds have probably been invoked to exclusively describe the cross-linking in slime is that they can create a so-called dynamic cross-linked network, meaning the cross-links are continuously made and broken. Some authors of older journal papers reasoned that covalent B–O ester bonds would be too strong to be dynamic and therefore couldn’t explain why slime is gooey and can be pulled apart easily.
Mahanthappa counters that rigorously peer-reviewed papers, including some based on 11B NMR analysis, strongly indicate that borate esters do the cross-linking and are weak enough to be dynamic.
The chemical inaccuracies discussed here don’t pertain only to fun and games with slime, so there’s even more reason to correct them. For example, petroleum engineering companies use borax cross-linking chemistry to produce hydraulic fracturing (fracking) fluids from guar gum, which reacts much the same way as PVA does but is less expensive. Their studies, some of which rely on high-pressure NMR techniques, also identify borate esters as the cross-linkers.
So where does all this leave chemistry educators and their slime demos? Michael D. Schaeberle, head of science at Middlesex School, in Concord, Mass., says if a fun demonstration sparks a young student’s interest in science, and as a result he or she learns about polymer cross-linking, it’s OK if subtle details and advanced concepts aren’t exactly right. “Maybe the student will go on to study science. And one day he or she will come up with the correct explanation for the way things work.” For C&EN’s readers, we’d like to set the record straight today.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter