Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
Neptunium can form double bonds with carbon
New neptunium complexes are helping chemists study periodic trends in f-block elements
by Ariana Remmel
June 8, 2022

It’s difficult to make molecules using elements that lie beyond uranium on the periodic table, which has hampered progress in studying how actinides form bonds. Now chemists have expanded the library of such molecules, creating a trio of transuranium complexes that contain double bonds between neptunium and carbon (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c02152).
“If you really want to understand actinide elements, you need to look at them across the period as far as you can go,” says Stephen Liddle, an inorganic chemist at the University of Manchester. But f-block elements tend to be more radioactive and difficult to aquire the heavier they get. Scientists have developed computational models that help mitigate the experimental bottleneck, but they need experimental data describing how transuranium elements behave in organometallic complexes, Liddle says. To address this gap, Liddle set his sights on the next element down the table from uranium, neptunium.
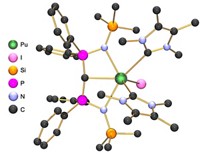
By choosing a set of phosphorus and carbene ligands known to form a variety of bond types with uranium, Liddle and his colleagues synthesized three new neptunium complexes and their cerium analogues. Their structural analysis of these transuranium molecules revealed the first experimentally observed dative single bonds and polar covalent double bonds between neptunium and carbon, Liddle says.
The research team used these newly validated molecular structures to predict bonding properties of thorium, uranium, and promethium analogues that are too unstable to synthesize. The new experimental data “enabled us to make actinide-actinide and actinide-lanthanide comparisons that would otherwise be really difficult,” Liddle says. He adds that this opens the door to a deeper understanding of periodic trends in bond properties across f-block elements grounded in real-world observations. The researchers plan to continue coaxing new bonding behaviors out of neptunium and other f-block elements. “This is just the beginning,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter