Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
New bonds predicted in actinide complexes
Phi and delta orbital interactions could improve nuclear waste management
by Sam Lemonick
April 9, 2020
| A version of this story appeared in
Volume 98, Issue 14

Separating heavy elements from one another is one of the biggest challenges of nuclear waste management. A new type of bonding between metals and ligands in actinide complexes opens new bonding possibilities that could help with these separations and improve nuclear waste processing (Nat. Commun. 2020, DOI: 10.1038/s41467-020-15197-w).
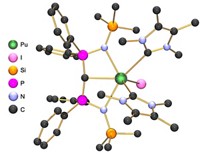
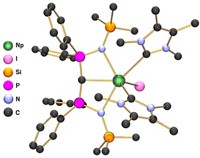
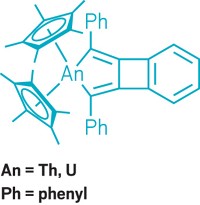
Actinide chemist Ping Yang and colleagues at Los Alamos National Laboratory calculated delta and phi bonding in actinide metallacycles that can change the strength of metal-ligand bonds in ways that could allow chemists to separate actinide elements from other heavy metals. Delta and phi bonds are covalent bonds involving four and six orbital lobes, respectively. They are known to occur between metals and ligands, but all previous examples looked very different. They involved either the ends of ligand p orbitals meeting the side of a fan-shaped metal f orbital, or the orbitals meeting end to end. In the last several years, other researchers synthesized thorium and uranium metallacycle complexes that put the metal and ligand atoms in the same plane, allowing for the ends of metal f orbitals to meet the sides of ligand p orbitals, or for the f and p orbitals to meet side to side, according to Yang’s group’s calculations.
To understand the effect of this new type of bonding on these compounds’ properties, Yang simulated the same metallacycle complexes with the actinide elements protactinium, neptunium, and uranium. Because actinide atomic radii get smaller moving left to right across the periodic group, metal-ligand bond lengths in actinide complexes typically decrease with increasing atomic number. But the researchers found that these new delta and phi interactions reverse that trend. For instance, in a cyclopropene complex, the distance between protactinium and the closest carbon atom is 2.26 Å, but substitute plutonium and the distance increases to 2.34 Å.
Yang explains that the shorter or longer bond lengths indicate stronger or weaker metal-ligand interactions in the different complexes. She thinks tuning these new delta and phi bonds could help chemists preferentially bind certain elements, something Yang says her group has been pursuing for a long time to help process nuclear waste. She also points out that because the phi and delta bonds only seem to be possible in 5f orbitals and not 4f orbitals because of the 5f’s bigger size, they could help separate actinide from lanthanide elements, which can contaminate nuclear material. “If we play this chemistry intelligently, we might design some ligands to improve separation in waste management,” Yang says.
Rebecca Abergel, a nuclear scientist at the University of California, Berkeley, and the Lawrence Berkeley National Laboratory, says the research could help in “long-standing problematic areas including separations, reprocessing, and waste management.” Her LBNL colleague, Stefan Minasian, says chemists might even use these interactions to make actinide-actinide bonds, which so far only exist in theory. Yang says she’s looking forward to seeing experimentalists synthesize these new delta and phi bonds.
CORRECTION
This article was updated on April 14, 2020, to correct the image credit. Credit should be to Morgan Kelley and Ivan Popov, not Ping Yang.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter