Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Bonding
The fight over the longest carbon-carbon bond is redefining what a bond is
Why chemists are pushing C–C bonds to their limits
by Laura Howes
March 9, 2019
| A version of this story appeared in
Volume 97, Issue 10

What is a carbon-carbon bond? You might think this is a question with a simple answer, but chemists are still working to figure it out. To do so, they are pushing carbon-carbon bonds to their very limits. Amid claims about who holds the record for longest bond, a more detailed story of bonding is emerging.
At Justus Liebig University Giessen, in Germany, Peter R. Schreiner admits that his research group has become a bit obsessed with bonding. The fascination took hold when the scientists stumbled upon a surprisingly long but stable carbon-carbon single bond. Their original aim, Schreiner says, was to synthesize artificial diamonds by stitching together carbon cages called diamondoids and “heating the hell out of them.” But in the process, they discovered the oddball bond, he says, and they became obsessed.
“I remember that very day in 2010 when my coworker came back with the X-ray structure of the product,” Schreiner says. The doctoral researcher in Schreiner’s lab had made a molecule with two diamantane cages linked by a carbon-carbon bond. The bond was much longer than normal.
Not only was the resulting bond long, but it was also surprisingly stable. The molecule didn’t dissociate until it was heated above 200 °C. Long bonds are usually weak bonds, so Schreiner predicted that the long carbon-carbon bond would break apart at a much lower temperature. “The first thing I thought was that something was wrong,” Schreiner recalls. “So I asked him to make even bigger and bigger ones—superlong bonds.” And that’s how the competition over ultralong bonds began.
Typical carbon-carbon bonds in alkanes measure 1.54 Å. Since 2011, one of Schreiner’s diamondoid compounds held the record for containing the longest single carbon-carbon bond (Nature 2011, DOI: 10.1038/nature10367). Its bond had a length of 1.704 Å. But once something is declared a record, people will certainly look to beat it. Two papers published in 2018 challenged Schreiner’s record and stretched the very idea of a carbon-carbon bond even further. But even though the new bonds are longer, they’re situated in very different molecules and thus in very different bonding environments. In some respects, the competing molecules are not comparable at all.
Building on Schreiner’s compound to make carbon-carbon bonds even longer would have been difficult because of a unique characteristic it has, says Takanori Suzuki of Hokkaido University. He explains that Schreiner’s carbon-carbon bond is a simple single bond, the kind found in alkane molecules such as ethane. But if the bond breaks between the cages in the compound, the molecule dissociates into two separate units, which adds entropy to the system. To stretch such a bond farther, Suzuki says, would mean balancing the dissociation energy of the long bond with the increase in entropy caused by breaking that bond.

So when Suzuki and his group set out to break Schreiner’s record, they wanted to make sure they reduced the entropy gain upon bond dissociation to increase their bond’s stability. Suzuki’s coworker Yusuke Ishigaki had the idea to stretch a carbon-carbon bond within a larger molecule so that if the bond breaks, the molecule doesn’t split into two. After some work, Ishigaki created a dihydropyracylene compound that broke the record. In a paper published last year, the team reported a bond length of 1.798 Å at –73 °C that expands to 1.806 Å at 123 °C (Chem2018,DOI: 10.1016/j.chempr.2018.01.011). In Suzuki and Ishigaki’s molecule, the chemically inert aromatic rings that surround the long carbon-carbon bond act like a shell that stabilizes the bond as it stretches farther and farther.
Just a few months after this team reported its feat, a group based in China announced it had made an even longer carbon-carbon bond (Angew. Chem., Int. Ed. 2018, DOI: 10.1002/anie.201812555).
During the synthesis and functionalization of 1,2-diamino-o-carboranes, Xu-Qiong Xiao’s group at Hangzhou Normal University found exceptionally long carbon-carbon bonds. “This was an unexpected finding,” Xiao says. “We became curious about it and started to investigate it in detail.” The bond lengths they reported topped out at 1.931 Å.

Although these record-breaking lengths are impressive, not everyone thinks the focus on one-upmanship among the groups is helpful. “One really doesn’t want to get into the phenomenon of boys comparing the length of their sexual equipment,” says Nobel laureate Roald Hoffmann of Cornell University. “You can quote me on that.”
“The strategies for elongating carbon-carbon bonds,” Hoffmann contends, “are more important for chemistry than the records.”
In fact, everyone C&EN spoke to for this story was clear that while all three groups had created long bonds between carbon nuclei, the bonding environments of the three molecules are different from one another. What is important about all three groups’ work, experts say, is how these long bonds were made and what they teach us about bonding itself.

Chemists know that covalent bonds are based on electrons shared between nuclei. Atomic nuclei stick together to form molecules because the nuclei are attracted to the negatively charged electrons on other atoms more strongly than they are repelled by the nuclei in those atoms. How the electrons are arranged around the nuclei, and how bonds are made and broken, is ultimately what controls chemistry.
Lines and arrows drawn on a page to show electron movement between atoms often describe what is happening in reaction flasks pretty well. But bonds aren’t quite as simple as these diagrams suggest. It is easy to visualize a carbon-carbon bond by drawing a line between two carbon atoms, says Anastassia Alexandrova, a specialist in electronic structure and chemical bonding at the University of California, Los Angeles. But, she says, the reality of bonding can become more complicated the more you study it.
“Every model [of bonding] is wrong,” she adds. “But some are useful, and that’s all that matters.”
A close look at Schreiner’s molecule reveals that it is stabilized by attractive dispersion forces known as London forces. These weak bonds exist between the hydrogen atoms on the cages on either side of the molecule, like two panes of glass that can stick together when touching face to face. Although individual dispersion interactions are weak, the effect is significant because there are so many hydrogen nuclei that can interact.
In the middle of the molecule, the ultralong carbon-carbon bond is essentially an alkane bond like the other carbon-carbon bonds within the cages of the molecule. That is not true of the unique carbon-carbon bonds that Suzuki’s and Xiao’s teams made, and that is why Schreiner still claims to hold the record for the longest alkane bond.
Ishigaki and Suzuki’s bond, for example, exists within a neutral hydrocarbon, just like Schreiner’s, but it is surrounded by aromatic rings and is not a straightforward alkane bond, as the researchers discovered in their study. In addition to using X-ray crystallography to determine the molecule’s structure, the team examined the bond’s character with Raman spectroscopy. That sort of analysis is something Hoffmann wishes more groups would do.
Instead of characterizing their unusually long carbon-carbon link as a pure alkane bond, the Japanese researchers describe it as “fuzzy.” It is, they say, a weak bond that is a mix of different quantum states.
The team’s unusual carbon-carbon bond might be weak, but it is still officially a bond and still longer than 1.803 Å, the shortest nonbonding distance between carbon nuclei calculated for caged dimer molecules. This theoretical limit is the length at which the bond dissociation energy is predicted to be zero. What Ishigaki has shown in this molecule—which can’t break apart if the carbon-carbon bond is severed—Suzuki says, is that “the bonded state and nonbonded state are connected seamlessly, in terms of the interatomic carbon-carbon distance.”
Meanwhile, carboranes and related molecules, like those studied by Xiao’s group, were already known to have inherently long, weak bonds. In fact, Hoffmann says he first measured carborane bond lengths himself when he was a grad student. The bonds in carboranes are typically long because the electrons within the cluster are often shared between more than two nuclei. The carbon-carbon bond in the Xiao group’s molecule is still a covalent bond, but its character is, again, not that of a simple alkane bond, because of the nitrogen and boron atoms also present in the molecule.
Xiao explains that the length of the remarkable carbon-carbon bond in his group’s cluster can be attributed to a delocalized nitrogen lone pair adding to the antibonding orbital of the special carbon-carbon bond. Xiao refers to this effect as negative hyperconjugation, whereas Hoffmann says he would use the term orbital interaction. Regardless of the name, “the analysis is pretty good,” Hoffmann says, “and I think it’s interesting that these bonds come out as long as they are.”
Suzuki agrees, adding that what matters is “not how long the bond is but how it was realized and how many new findings there are.”
Advertisement
And that underscores the important point about this work. UCLA’s Alexandrova is one of the organizers of an annual conference on chemical bonding, which brings together researchers to explore the nature of the chemical bond. The forum still exists, she explains, because this discussion continues.
These three groups are working on distinct aspects of bonding and studying very different types of chemical systems. But they are all focusing on carbon-carbon bonds to understand bonding better. At the same time, they hope that what they learn will one day be useful.
Schreiner, for example, describes his primary motivation for continuing to pull at carbon bonds as simple curiosity. But he hopes to apply what he learns to his work on catalysis and transition states. Other researchers suggest that understanding these extreme bonding situations could help design novel functional materials or new chelating ligands.
But while there is hope that these bonding insights might find practical use, Hoffmann says he would make a case for continuing the work purely from an intellectual standpoint. “I think testing the limits gives you a better understanding of the concept,” he says, adding that these researchers are “enriching our understanding of the carbon-carbon bond.”
To understand the nature of covalent bonds, chemists have to understand extreme cases. And one of those extremes is length. In Hoffmann’s view, extreme cases can teach us about molecules with typical bond lengths. They can also be used like paradoxes, to help us interrogate basic chemistry assumptions, he adds.
“I think it’s nice that we’re stretching or breaking some general chemistry rules,” Alexandrova says. “It’s something that I could even use in my class.” Xiao agrees: “The carbon-carbon bond is a well-accepted concept that we teach every undergraduate student in freshman courses,” he says. But the exact definition remains elusive.
These extremes may nudge chemists toward a clearer understanding of molecular structure, but chemical bonding is not a black-and-white concept. Rather, as Schreiner puts it, “it’s all a gray zone.”
UPDATE: April 17, 2019
After this article was first published, a few researchers contacted C&EN to let us know that even longer carbon-carbon bonds exist than the ones we mentioned. And these bonds predate some of the ones we highlighted. So C&EN has decided to stretch this article, like the bonds, even further to include some superlative examples.
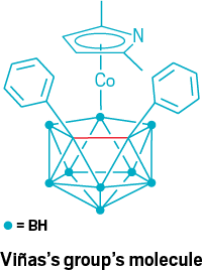 In 2002, a team including Clara Viñas of the Institute of Materials Science of Barcelona (ICMAB-CSIC) described a 2.022 Å long carbon-carbon bond in an inorganic cluster (Inorg. Chem. 2002, DOI: 10.1021/ic011285z). Like Xiao’s 1.931 Å bond, mentioned in our original story, this bond is found in a carborane cluster. Unlike Xiao’s bond, though, this one is part of a carborane within a larger organometallic compound. The compound features a negatively charged dicarbollide coordinated to a pyrrolyl anion via a cobalt(III) ion. As discussed in our story, the bonds in carborane clusters are more diffuse than a simple two-center, two-electron bond, and this holds true for Viñas’s bond as well. When asked to describe their bond, Viñas and group leader Francesc Teixidor describe how a combination of sterics and back electron donation to an antibonding orbital create a long carbon-carbon distance that has fewer electrons than a simple single bond between carbons. Viñas also mentions an even older paper, from 1987, in which Ken Wade, then at Durham University, and colleagues reported a carbon-carbon distance of around 2.001 Å in a carborane (J. Chem. Soc., Chem. Commun. 1987, DOI: 10.1039/c39870000889). This find in the literature shows that investigations pushing carbon-carbon bonds to their extremes have been going on for longer than our article indicated.
In 2002, a team including Clara Viñas of the Institute of Materials Science of Barcelona (ICMAB-CSIC) described a 2.022 Å long carbon-carbon bond in an inorganic cluster (Inorg. Chem. 2002, DOI: 10.1021/ic011285z). Like Xiao’s 1.931 Å bond, mentioned in our original story, this bond is found in a carborane cluster. Unlike Xiao’s bond, though, this one is part of a carborane within a larger organometallic compound. The compound features a negatively charged dicarbollide coordinated to a pyrrolyl anion via a cobalt(III) ion. As discussed in our story, the bonds in carborane clusters are more diffuse than a simple two-center, two-electron bond, and this holds true for Viñas’s bond as well. When asked to describe their bond, Viñas and group leader Francesc Teixidor describe how a combination of sterics and back electron donation to an antibonding orbital create a long carbon-carbon distance that has fewer electrons than a simple single bond between carbons. Viñas also mentions an even older paper, from 1987, in which Ken Wade, then at Durham University, and colleagues reported a carbon-carbon distance of around 2.001 Å in a carborane (J. Chem. Soc., Chem. Commun. 1987, DOI: 10.1039/c39870000889). This find in the literature shows that investigations pushing carbon-carbon bonds to their extremes have been going on for longer than our article indicated.
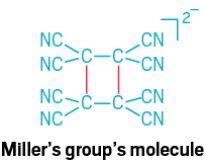 An even longer, and definitely more diffuse, carbon-carbon bond can be found in a molecular system studied by Joel S. Miller and his group at the University of Utah. In 2001, Miller’s team discovered the bond in crystal structures of a transition-metal complex that included the radical anion tetracyanoethylene (TCNE). Two TCNE units had formed a dimer in which the central carbons are separated by 2.9 Å. Although this bond is σ in character, it is formed by the overlap of the two antibonding π systems on the TCNE monomers (Angew. Chem., Int. Ed. 2001, DOI: 10.1002/1521-3773(20010702)40:13<2540::AID-ANIE2540>3.0.CO;2-O). Miller’s team confirmed this four-atom, two-electron bond with spectroscopic evidence in 2013. The group has also described an eight-atom, two-electron carbon-carbon bond that’s 3.14 Å long (J. Am. Chem. Soc. 2009, DOI: 10.1021/ja902790q).
An even longer, and definitely more diffuse, carbon-carbon bond can be found in a molecular system studied by Joel S. Miller and his group at the University of Utah. In 2001, Miller’s team discovered the bond in crystal structures of a transition-metal complex that included the radical anion tetracyanoethylene (TCNE). Two TCNE units had formed a dimer in which the central carbons are separated by 2.9 Å. Although this bond is σ in character, it is formed by the overlap of the two antibonding π systems on the TCNE monomers (Angew. Chem., Int. Ed. 2001, DOI: 10.1002/1521-3773(20010702)40:13<2540::AID-ANIE2540>3.0.CO;2-O). Miller’s team confirmed this four-atom, two-electron bond with spectroscopic evidence in 2013. The group has also described an eight-atom, two-electron carbon-carbon bond that’s 3.14 Å long (J. Am. Chem. Soc. 2009, DOI: 10.1021/ja902790q).
Can these charged molecules, with their diffuse bonds, be compared to simpler carbon-carbon σ bonds like Schreiner’s, mentioned in our original story, when they have such different bonding environments? “In my opinion,” says Alexandrova, who commented on the bonds in our original article, “it is an unfair comparison.” What is true, she says, is that these molecules have bonded interactions between carbon atoms and that the carbon-carbon distances are unusually long. “These are,” Alexandrova concludes, “beautiful bonds.”
Readers, if you know of any other impressive carbon-carbon bonds, add them in the comments below.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter