Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Computational Chemistry
Machine learning solves a long-standing DFT problem
A neural network makes more accurate density functional theory predictions about electron sharing than do equations written by humans
by Sam Lemonick
December 10, 2021

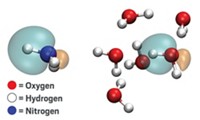
Knowing where the electrons are within a molecule can go a long way to explaining its structure, its properties, and its reactivity. Chemists use density functional theory (DFT) methods, approximations of the exact distribution of a molecule’s electrons, to make accurate and computationally efficient models of molecules and materials. But there are well-known circumstances where DFT tools fail. One is predicting how atoms share electrons; in one famous example, DFT methods incorrectly predict that even when a chlorine and a sodium atom are infinitely far apart, the chlorine atom retains a fraction of one of the sodium atom’s electrons.
Errors like that arise because DFT equations are scientists’ approximations of physical reality. Researchers associated with Alphabet Inc.’s DeepMind machine learning project say that their neural network eliminates that electron error and makes more accurate predictions than traditional DFT methods (Science 2021, DOI: 10.1126/science.abj6511).
The group trained their neural network on data about the properties of small, main-group models and on imaginary systems that contain fractions of electrons that previous work had theorized might help resolve the known DFT failures (Science 2008, DOI: 10.1126/science.1158722). They then used the resulting neural network, called DeepMind21 (DM21) to predict electron distributions for test molecules. DM21 made more accurate calculations than standard DFT programs for these test systems, which included an adenine-thymine DNA base pair and a chain of hydrogen atoms. In some cases, DM21’s calculations came close to those resulting from much more computationally-intensive methods, which are a gold standard for comparison but impractical for everyday computational use.
James Kirkpatrick of DeepMind says the standard DFT approach is for a computational chemist to write a system of equations that incorporates a few known mathematical properties of molecules. Those limited constraints lead to errors, like the electron sharing example, in certain situations. The neural network, on the other hand, is simply interpolating a set of equations from the data it has seen, without any preconceived ideas about mathematical properties of molecules.
With the new work, the researchers have proved the success of that approach, says John P. Perdew, a Temple University physicist whose work over several decades has laid the foundations for progress on DFT methods.
DFT researcher Kieron Burke of the University of California, Irvine says that while this approach requires a lot of expertise, time, and computing power, if DM21 proves it can outperform other approaches in more situations and doesn’t require much computing power, the team may have hit on “a whole new way to create DFT approximations.” He says it remains to be seen how DM21 performs on other systems, like transition metal complexes and non-bonding interactions.
Franklin says the group plans to share DM21 with the public, as well as its underlying code and software to allow users to incorporate it into existing programs. And he says the team has started to explore some of the systems Burke mentioned.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter