Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Geochemistry
Video: Why the glowing rocks under New Jersey fascinate geochemists
The complex mineral collection beneath northern New Jersey may offer clues to how Earth formed and how soil affects water purity and plant growth
by Kerri Jansen
February 12, 2020
| A version of this story appeared in
Volume 98, Issue 7
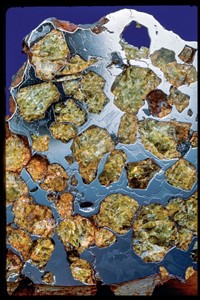
The Franklin–Sterling Hill area of New Jersey is home to a prized mineral deposit that has fascinated geochemists and collectors for decades—not least because many of the minerals are vibrantly fluorescent. In this episode of Speaking of Chemistry, Kerri Jansen visits the Sterling Hill Mining Museum and the National Gem and Mineral Collection to learn why New Jersey’s rocks are so special and what scientists hope to learn from the variety of minerals in the collection, fluorescent or not.
Subscribe to our YouTube channel to catch all our chemistry news videos.
The following is the script for the video. We have edited the interviews within for length and clarity.
Kerri Jansen: There’s something special about the rocks underneath the small town of Ogdensburg, New Jersey. They glow. More precisely, they fluoresce. That fluorescence arises from the unusual geochemistry of this area, which has fascinated collectors and scientists for decades. We visited the Sterling Hill Mining Museum in Ogdensburg to find out more.
The museum houses artifacts from the area’s zinc-mining history, along with an expansive collection of prized mineral specimens. That some of them glow is just a bonus. They draw visitors in. And once here, those visitors learn about the area’s importance to mineral science. Scientists have identified nearly 400 different mineral species in the area, which is often referred to as the Franklin–Sterling Hill region. In fact, some haven’t been found anywhere else in the world. There’s such an exceptional variety and abundance of minerals that Jeff Post, curator of the National Gem and Mineral Collection in Washington, DC, called it “the mineral equivalent of a tropical rain forest.”
Jeffrey Post: Franklin–Sterling Hill is just an incredible diversity of minerals—really hundreds of different minerals have come out of that deposit. And so the chemistry is very interesting, very complicated.
Kerri: The chemistry is so interesting that these New Jersey minerals show up in collections all over the world—including several thousand specimens in the collection Jeff oversees, which is located at the National Museum of Natural History. I volunteer at that museum, although I’d never met Jeff or seen his amazing collection of minerals before.
Jeffrey Post: We could go into almost every aisle in this room and start opening some drawers, and we’d hit places where suddenly you’re seeing New Jersey, New Jersey, New Jersey. This was a collection that we wanted to build up because we know long into the future, scientists will continue to be interested in this really unusual mineral deposit.
Kerri: We wanted to understand what makes the minerals under New Jersey so special and how they’ve come to hold such incredible importance to scientists.
To do that, we visited the minerals at their source, the old mine tunnels underneath Sterling Hill.
Of the nearly 400 mineral species found here, about 90 fluoresce in a variety of colors.
Earl Verbeek: You see we have some willemite here?
Kerri: It looks like someone flicked a highlighter at the wall.
Earl Verbeek: It does.
Kerri: The voice you just heard belongs to Earl Verbeek, the Sterling Hill Mining Museum’s resident geologist and our tour guide for this geochemical expedition.
The willemite he just pointed out is a mineral that contains zinc. It was one of the main ores that made the site prosperous for decades until its closure in 1986.
Earl Verbeek: So wherever you see green like that, that’s willemite, and you know you’re in, are very near the ore body.
Kerri: We also saw a lot of calcite, which produces an orangish-red color.
Earl Verbeek: That’s what the miners did not want to see. It’s pretty, but it’s worthless.
Kerri: Both minerals get their glow on because of a little bit of manganese that has snuck into their mineral structures. Not all minerals fluoresce, and among the ones that do, the fluorescence is often caused by an impurity. Different mineral structures and compositions can produce different colors and color intensities, information that helped miners find their way to the richest ore deposits.
But the flashiest minerals found in Sterling Hill aren’t necessarily the most interesting. Back in Washington, Jeff Post is studying manganese oxide minerals from Franklin–Sterling Hill. These specimens aren’t nearly as photogenic as their fluorescent counterparts—in fact, some scientists have nicknamed these dingy black and brown oxides “manganese mud.” But don’t let their appearance fool you. Jeff says these minerals have an important role on earth.
Jeffrey Post: These manganese oxides can act as scavengers. They can suck up all kinds of heavy metals and bad things that might be a problem in the environment. Or they can act as catalysts—they can cause other chemical reactions to take place.
Kerri: So Jeff wants to understand how these minerals, which appear in soils all over the world, may be affecting water purity or how plants grow. The diverse collection from Franklin–Sterling Hill is a rich resource for that work.
Jeffrey Post: The earth has had 4½ billion years of chemistry to make all kinds of things.
And there’s probably no more complicated laboratory experiment ever done in the earth that we know of than in Franklin–Sterling Hill.
Kerri: As Earl Verbeek told me, scientists know in general terms how the complicated deposits under Sterling Hill formed, but there are a lot of geochemical blanks to be filled in. For Franklin–Sterling Hill, the story starts about a billion years ago, when what is now New Jersey was underwater.
Submarine eruption spewed out metal-rich fluids that reacted with oxygen in seawater to create oxide minerals. Over time, these minerals were cooked and squeezed into the menagerie of minerals we see today, from the dull manganese muds to the brilliant fluorescent specimens. Exactly how all that cooking and squeezing went down is a mystery that researchers are still trying to figure out. Scientists like Jeff Post and others are also interested in learning what the Franklin–Sterling Hill minerals can tell them about how the earth’s complex geological processes work today.
Earl Verbeek and the folks at the Sterling Hill Mining Museum are supporting that effort, with the help of some newly acquired analytical equipment. They’re using instruments like this Raman spectrometer to analyze their mineral specimens. This instrument provides a molecular fingerprint, which helps ID a substance. Traditional visual ID methods are pretty good for identifying a mineral, but they’re not infallible.
Earl Verbeek: We don’t want to be saddled only with educated guesses. Sometimes we’re wrong.
Kerri: In fact, Earl found that some specimens in Sterling Hill’s collection had been misidentified. This one, for example, contains a white mineral originally identified as datolite, a silicate mineral containing calcium and boron, but turned out to be barite—barium sulfate. Having the right ID is important to mineralogists as well as collectors. As Earl notes, it could mean the difference between a $5 rock and a $5,000 one.
Earl hopes these analytical experiments will provide enough data to understand the complex mix of trace minerals at Franklin–Sterling Hill—what they are and where they come from.
Kerri: And in the meantime, these exceptional minerals are still available for everyone, mineralogists or not, to enjoy.
Kerri: Woooooaahhh.
Earl Verbeek: So you can see the red here is calcite.
Kerri: Yeah, my brain is telling me that you’re just shining a red light on it, but you’re not.
Earl Verbeek: No.
[laughter]
Kerri: If you’d like to learn more about the Sterling Hill Mining Museum or the National Gem and Mineral Collection, check out the links we’ve shared on C&EN’s website. And if there’s something you love about minerals, please let us know in the comments.
Fin.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter