Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Surface Chemistry
Mechanisms for catalytic CO oxidation on platinum found at last
New surface chemistry method answers mechanistic questions that have eluded chemists for 40 years
by Stu Borman
June 14, 2018
| A version of this story appeared in
Volume 96, Issue 25
A new surface chemistry technique has allowed researchers to determine the long-sought mechanisms of one of the most studied reactions in heterogeneous catalysis: the oxidation of carbon monoxide on a platinum surface.
Catalytic CO oxidation cleans up emissions from motor vehicles and industrial smokestacks. Studied for about 40 years, it is a textbook system for the experimental and theoretical understanding of heterogeneous oxidations—gaseous reactions on catalytic surfaces.
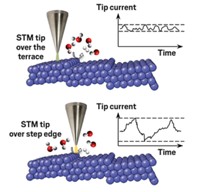
The reaction is complex. Pt surfaces have two possible active sites. About 99% of each surface is flat terraces with moderate catalytic activity, and the other 1% is highly active steps between layers of Pt atoms. And the reaction produces two types of CO2 molecules: hyperthermal, with high energies and velocities, and thermal, with moderate energies and speeds.
Scientists have lacked analytical tools to unpack these complexities, so the exact mechanisms by which CO gets oxidized have been a mystery. The consensus view was that a single type of reaction on terrace sites produced both hyperthermal and thermal CO2.
Theofanis N. Kitsopoulos at the Max Planck Institute for Biophysical Chemistry and coworkers now provide a more nuanced view (Nature 2018, DOI: 10.1038/s41586-018-0188-x). They used a molecular beam to shoot CO at an O2-bound Pt surface at precise times, and then employed slice-ion imaging to determine the reaction site, speed, and escape angle for specific CO2 molecules flying off the surface. These tools had not been used together before to measure the speed and direction of surface-generated species.
The study shows that the reaction has three mechanisms. Two produce thermal CO2 and dominate at temperatures below about 700 K: Either CO on terrace sites diffuses to a step and reacts there with an oxygen atom, or CO already on a step reacts with O. In the third mechanism, which predominates at high temperatures, terrace CO reacts with terrace O to produce hyperthermal CO2.
The results “are phenomenal,” says surface chemist Dan Killelea of Loyola University Chicago. “The ability to experimentally distinguish among different possible catalytic reaction sites on a surface is a long-sought goal” that the new approach makes possible.
The strategy could be applied to other heterogeneous surface reactions as well. “It has the potential to shift the entire focus of efforts to understand mechanisms of heterogeneously catalyzed chemical reactions,” Killelea says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter