Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Consumer Safety
Covid-19
A chemist’s guide to disinfectants
Has your local store run out of sanitizing wipes? This cheat sheet can help you find and understand alternatives
by Craig A. Bettenhausen
May 1, 2020

“You’re a chemist, right? We’re almost out of wipes. Do you have any ideas on what else we could use to disinfect?” As supplies in grocery store cleaning aisles dwindle, chemists and other people with science backgrounds are fielding questions like these from friends and relatives about what they can use to kill the novel coronavirus, SARS-CoV-2.
Although manufacturers of disinfectants are doing all they can to keep up, demand is through the roof, and some raw material supply chains are strained. So how should you advise your friends and family on their available options?
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe
C&EN constructed this guide to explain the ingredients in disinfectants and help you give good advice. The most important thing is to read the labels. The US Environmental Protection Agency regulates disinfectants used on hard and soft surfaces under its authority to regulate pesticides. The agency vets and stands behind the efficacy promises on the labels, as long as you follow the instructions.
Labels offer guidance on how to use disinfectants safely—for instance, in a ventilated area—and they explain which cleaning products shouldn’t be mixed with other chemicals. Interactions you might not expect can create toxic gases or make the mixture stronger or weaker than anticipated.
Even the type of cloth you use when cleaning hard surfaces might alter how a disinfectant works. For instance, paper towels can decompose after long soaks in some disinfectants and deactivate others. The fabric in wipes is specially formulated to be unreactive, so experts advise that you don’t try to make your own premoistened wipes. Instead, you should spray a liquid disinfectant onto the target surface, let it sit for at least the “dwell” or “contact” time listed on the label, and then wipe dry or let the liquid evaporate. For soft surfaces like cloth or food, experts suggest different cleaning methods.
SARS-CoV-2 is an enveloped virus, which means it is surrounded by a lipid membrane. That’s good news, because a wide variety of disinfectants can disrupt lipid membranes, killing the virus they were protecting. Few disinfectants have been rigorously tested against SARS-CoV-2 in the lab, but the EPA maintains a public database of products it recommends for use against SARS-CoV-2 on the basis of their proven efficacy against similar viruses. Users can search this list by product name, active ingredient, type of product, and more. Users can search EPA's database, called List N, by product name, active ingredient, type of product, and more.
Disinfectant wipes and sprays used to clean hard surfaces are currently scarce, so we’ve curated the list below to describe the chemicals used in those products. You can use this information as a cheat sheet while you read the labels on the products you can find.
-
Alcohols
How you’ll see them
 Ethanol
Ethanol
(ethyl alcohol)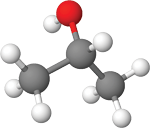 Isopropanol
Isopropanol
(isopropyl alcohol or 2-propanol)How they work
By disrupting a virus’s lipid envelope or by clumping or denaturing its proteinsWet contact time needed*
1–5 minutesUse notes
Ensure adequate ventilation and wear glovesSafety concerns
Flammable, poison risk upon ingestion and can damage plastics and cause heady fumesFound in
 Hand
Hand
sanitizers Wipes
Wipes -
Reducers
How you’ll see them
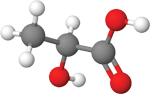 ʟ-lactic acid
ʟ-lactic acid Citric acid
Citric acidHow they work
By denaturing a virus’s proteins, disrupting its lipid envelope, and reducing critical viral componentsWet contact time needed*
5 minutesUse notes
Wear glovesSafety concerns
Generally recognized as safe, though they can irritate skinFound in
 Sprays
Sprays -
Oxidizers
How you’ll see them
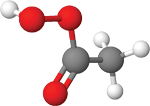 Peracetic
Peracetic
acid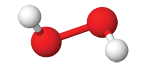 Hydrogen
Hydrogen
peroxideNaClO
Bleach
(sodium hypochlorite)How they work
By denaturing a virus’s proteins, disrupting its lipid envelope, and oxidizing sulfur bonds in proteins, enzymes, and other metabolitesWet contact time needed*
Bleach, 1 minute; hydrogen peroxide, 5 minutes; peracetic acid, 2–5 minutesUse notes
Ensure adequate ventilation and wear glovesSafety concerns
Can irritate skin, mucus membranes, and airways and can damage clothingFound in
 Wipes
Wipes Sprays
Sprays Concentrates
Concentrates -
Quaternary ammonium salts
How you’ll see them
 Alkyldimethylbenzylammonium chloride
Alkyldimethylbenzylammonium chloride
(Benzalkonium chloride) Octyl decyl dimethylammonium chloride
Octyl decyl dimethylammonium chlorideHow they work
By removing a virus’s lipid envelope, denaturing its proteins, and disrupting its enzymesWet contact time needed*
10 minutesUse notes
Deactivated by hard water and fabric; wear glovesSafety concerns
Can irritate the skinFound in
 Wipes
Wipes Sprays
Sprays Concentrates
Concentrates Antibacterial
Antibacterial
hand soap*The time the disinfectant should sit on a surface to ensure it works before being wiped away
Sources: US Environmental Protection Agency; Ryan Cotroneo/UNX Industries; Steven Bennett/Household and Commercial Products Association; Kevin Coyne/Wexford Labs; J. Vet. Med. Sci. 2000, DOI: 10.1292/jvms.62.85; Kirsten M. Thompson, “The Science of Disinfectants,” Cleaning and Maintenance Management, April 25, 2012; ScienceDirect.
Note: To sanitize soft surfaces such as clothes and food, use these tried-and-true methods. To disinfect textiles like your clothes, running them through the laundry with detergent is the best choice. For your hands, soap is the best tool, and hand sanitizer is a good on-the-go alternative if you’re not near a sink. For fruits and vegetables, the US Department of Health and Human Services says to cut away any damaged or bruised areas, then rinse under running water without soap, bleach, or commercial produce washes. The agency recommends you don’t wash bagged produce marked “prewashed” or meat, poultry, or eggs.
CORRECTION
This story was updated on May 2, 2020, because the labels for hydrogen peroxide, ʟ-lactic acid, and peracetic acid did not appear with the corresponding structures. The structures have been moved accordingly.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter