Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Consumer Safety
More evidence that sunscreens absorb through skin
US FDA finds 6 active ingredients in blood plasma of study participants
by Britt E. Erickson
January 22, 2020
| A version of this story appeared in
Volume 98, Issue 4
The US Food and Drug Administration has confirmed that six active ingredients widely found in sunscreens penetrate through the skin and absorb into blood plasma. The agency’s findings, published on Jan. 21, put pressure on manufacturers to determine whether such exposure to sunscreen ingredients is safe (JAMA 2020, DOI: 10.1001/jama.2019.20747).






“The fact that an ingredient is absorbed through the skin and into the body does not mean that the ingredient is unsafe,” Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, says in a statement. “Rather, this finding calls for further industry testing to determine the safety and effect of systemic exposure of sunscreen ingredients, especially with chronic use.”
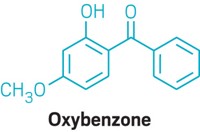
The FDA has yet to finalize a rule proposed in February 2019 that would require sunscreen manufacturers to provide safety data if their products contain certain ingredients, so that the agency can evaluate whether the chemicals are generally recognized as safe and effective. Those ingredients include the six chemicals—avobenzone, homosalate, octinoxate, octisalate, octocrylene, and oxybenzone—the FDA tested in its new study. The FDA wants the additional data because of increased use of sunscreens and potential risks.
The latest study follows up on earlier FDA work that found that four sunscreen active ingredients absorb through the skin (JAMA 2019, DOI: 10.1001/jama.2019.5586). Of the six compounds evaluated in the new study, three had been assessed previously and three were new. The FDA tested various formulations—lotion, aerosol spray, nonaerosol spray, and pump spray—on more people than the first study. After a single application, all six active ingredients in all tested formulations produced levels of the active ingredient in participants’ blood plasma greater than 0.5 ng/mL, the FDA’s threshold for potentially waiving safety studies.
The Personal Care Products Council and the Consumer Healthcare Products Association, which represent sunscreen manufacturers, noted in a statement that “there were no serious drug-related adverse events reported in the trial, consistent with the excellent safety record associated with sunscreen active ingredients over decades of real-world use.”
While the industry conducts further testing, the FDA is advising consumers to continue using sunscreens in conjunction with other measures, such as wearing protective clothing, to reduce the risk of skin cancer.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter