The electric-powered future with new cathode materials
With the aim to curb air pollution and address climate change, a number of European countries have announced plans to ban sales of new cars containing internal combustion engines over the next 25 years. Norway has set the most ambitious deadline of 2025.
Electric vehicles have the potential to fill the void left by diesel- and gasoline-powered cars. Thanks to advances in battery technology and manufacturing, which have steadily reduced costs over the past decade, batteries have “the ability, not just on the vehicle side, but also on the stationary side, to actually change the energy landscape quite a bit,” says Eric Dufek, the energy storage group lead at Idaho National Laboratory.
Yet more development is needed, particularly in regard to the electrode materials in the battery, to ensure that electric vehicles are widely adopted. The International Energy Agency reported that the global number of electric passenger cars increased from 2 million to 3.1 million in 2017. While this growth rate is impressive, electric cars account for only about 0.3% of the more than a billion passenger vehicles on the road today.
Most consumers are waiting for electric vehicles with greater range, faster charging times, and price tags comparable to combustion-engine cars—all features tied to the battery. Consequently, the U.S. Department of Energy has set goals to increase range to 300 miles, decrease charging time to less than 15 minutes, and reduce the cost of electric-vehicle batteries to less than $100/kWh.
A battery pack that costs $100/kWh or less will enable electric vehicles to be cost competitive with internal combustion engine vehicles without the aid of government subsidies, says David Howell, the deputy director for the U.S. Department of Energy’s Vehicle Technologies Office.
Developing better battery materials will play an important role in reaching the $100/kWh goal. However, the process is complicated because five characteristics must be optimized simultaneously: energy density, which relates to vehicle range; power, which affects acceleration and charging properties; lifetime; cost of raw materials; and safety. “All five of those characteristics depend on the cathode material,” says Alan Nelson, chief technology officer and sector chief executive for new markets at Johnson Matthey, a global leader in science that enables a cleaner and healthier world. “If you ask what’s really limiting the performance of a battery, today, it’s principally that cathode material.”
Today’s lithium-ion cathodes are intercalation materials, meaning their structure possesses channels filled with lithium ions. As the battery charges and discharges, some of these ions flow back and forth between the two electrodes. Lithium cobalt oxide debuted as the first intercalation cathode in 1991 and is still used in consumer electronics. However, lithium cobalt oxide’s electrochemical properties are inadequate for electric vehicle applications, and cobalt is a limited and expensive component. “You want to reduce cobalt to reduce the cost of the cell,” Howell explains.
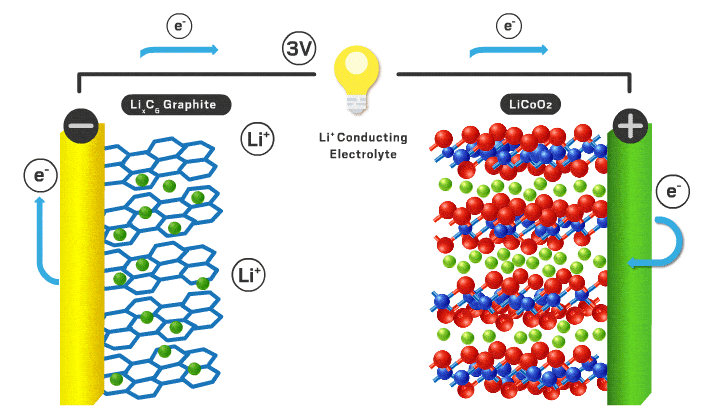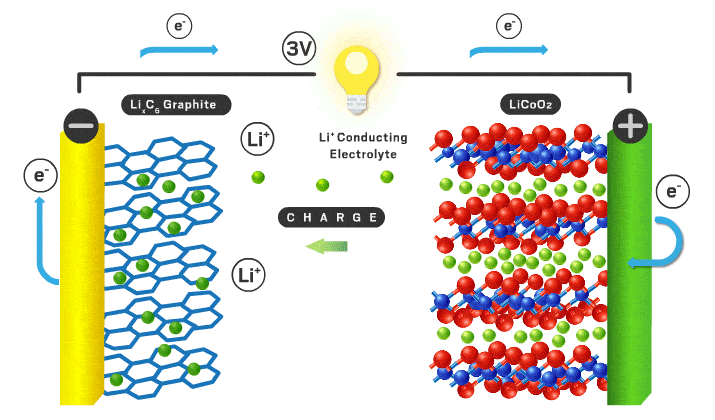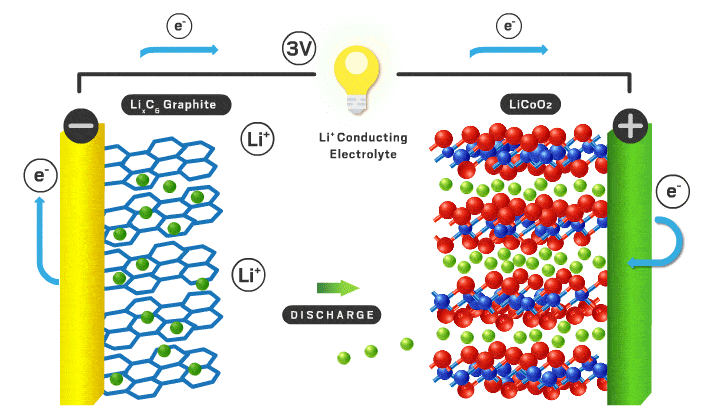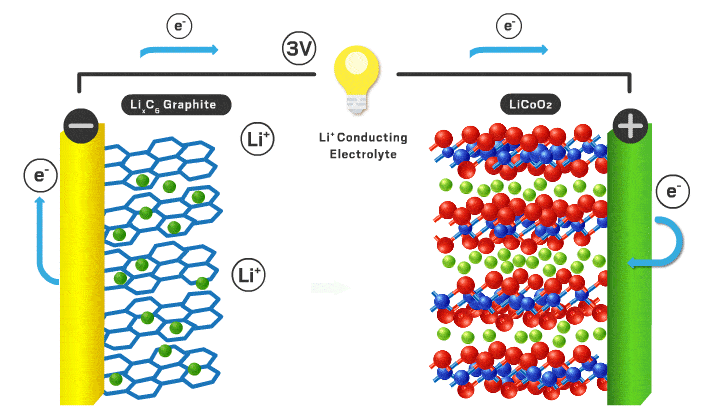
Over time, scientists found that incorporating nickel increases energy density because it allows more lithium ions to leave the cathode without compromising the structure of the material. Nickel is also cheaper than cobalt. Nickel’s appeal has resulted in the nickel manganese cobalt (NMC) and nickel cobalt aluminum materials that are used in modern electric vehicles. Numbers that follow the acronyms give the ratio of the different metals. For example, NMC 111 contains equal amounts of nickel, manganese, and cobalt. But high-nickel materials tend to lose stability, which can compromise lifetime and safety.
Johnson Matthey, a company also known for catalysts for automotive emission control and chemical processing, as well as a range of other science-led sustainable technologies, has developed a family of promising new cathode materials called eLNO™, which show improved performance compared to other high-nickel materials. The energy density of eLNO is 20–25% higher than NMC 622, the current commercial benchmark, and 5–10% higher than next-generation NMC 811. “Importantly, it’s not just energy density,” Nelson says. “We’re doing that while maintaining the other characteristics like lifetime and safety.”
Although the eLNO materials contain some cobalt and other elements, they have less cobalt than NMC 811. Researchers are hunting for ways to remove even more.
Johnson Matthey has announced plans to scale up production of eLNO to 10,000 metric tons per year by 2021–2022. In the meantime, Nelson emphasizes the importance of continuing to study cathode materials in order to understand their behavior at a fundamental level. He says, “Then you can design and tune them to do exactly what you want them to do in real-world applications.”
