Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Bismuth, often overlooked in the periodic table, breaks into redox catalysis
Main-group element acts like a transition metal in fluorination reaction
by Bethany Halford
January 16, 2020
| A version of this story appeared in
Volume 98, Issue 3
Think of bismuth as the Cinderella of the periodic table. Because it is surrounded by its toxic stepsiblings—the elements lead, tin, antimony, tellurium, and polonium—chemists often overlook bismuth as a possible reagent. But bismuth and its compounds are safe enough to use in pharmaceuticals, in cosmetics, and as a replacement for lead materials in plumbing systems. Now, chemists at the Max Planck Institute for Kohlenforschung are giving bismuth a chance to shine, plucking it from the periodic table and fashioning it into a redox complex that mimics the actions of a transition-metal catalytic cycle.
“Our idea was: If transition metals have been so successful following certain rules, can we basically take bismuth and make it behave like a transition metal?” explains Josep Cornella, who led the research project.
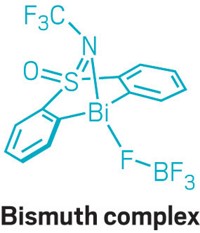
But bismuth has some peculiar properties. It can coordinate up to nine ligands and adopt geometries that differ from those of transition metals. Still, Cornella’s team was able to design and build a bismuth catalyst (shown) that fluorinates aryl boronic acids and esters through a Bi(III)/Bi(V) redox cycle (Science 2020, DOI: 10.1126/science.aaz2258).
“This is a reaction that would be very difficult to discover by just mixing and stirring and doing high-throughput screening,” Cornella says. “There is a whole mechanistic rationale behind it.”
Cornella doesn’t expect the fluorination to replace existing methods for this reaction. Rather, he sees it as a proof-of-concept demonstration of what bismuth can do. “We don’t want to replace transition metals. We would like to provide transformations that transition metals are not able to do,” he says. “This is the tip of the iceberg for us.”
“This work should be considered one of the most important advances in molecular bismuth chemistry and a landmark in the field of main-group chemistry more generally,” says Saurabh S. Chitnis, an expert in main-group chemistry at Dalhousie University. Chitnis points out that chemists currently have to use harsh oxidants to drive the bismuth catalytic cycle but that this is often the case with heavy-element redox processes. “Nevertheless, a glimpse at such remarkable reactivity provides a compelling argument for further exploring the fundamental coordination and redox chemistry of heavy main-group elements: there’s plenty of room at the bottom of the periodic table.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter