Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
C&EN’s molecules of the year for 2020
Our editors highlight the coolest molecules unrelated to COVID-19 that were reported this year
by Celia Henry Arnaud
December 8, 2020
| A version of this story appeared in
Volume 98, Issue 48
Poll: Our readers have voted for their favorite molecule of 2020. The winner is Porous ionic liquid
| Molecule | Votes | Percentage |
| Porous ionic liquid | 292 | 28.4% |
Largest aromatic ring |
181 |
17.6% |
| Asymmetrical molecular knots | 178 | 17.3% |
2-D metallo-supramolecule |
143 |
13.9% |
Boron quadruple bond |
126 |
12.3% |
Threading rings on a polymer chain |
80 |
7.8% |
| Beryllium radical cation | 27 | 2.6% |
Molecule: Porous ionic liquid
Votes: 292
Percentage: 28.4%
Molecule: Largest aromatic ring
Votes: 181
Percentage: 17.6%
Molecule: Asymmetrical molecular knots
Votes: 178
Percentage: 17.3%
Molecule: 2-D metallo-supramolecule
Votes: 143
Percentage: 13.9%
Molecule: Boron quadruple bond
Votes: 126
Percentage: 12.3%
Molecule: Threading rings on a polymer chain
Votes: 80
Percentage: 7.8%
Molecule: Beryllium radical cation
Votes: 27
Percentage: 2.6%
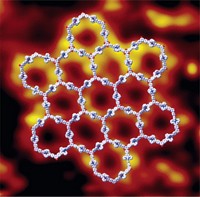
Largest aromatic ring to date synthesized

Year in chemistry 2020
University of Oxford researchers constructed a 16 nm wide molecular wheel, which contains 12 porphyrins and 162 π electrons. In the ring, zinc porphyrins are linked by alkynes and held in place by molecular spokes. The molecule is of interest for quantum computing (Nat. Chem. 2020, DOI: 10.1038/s41557-019-0398-3).
Huge 2-D metallo-supramolecule assembled

A multi-institution team made a 2-D metallo-supramolecule that measures nearly 20 nm across (Nat. Chem. 2020, DOI: 10.1038/s41557-020-0454-z). Ruthenium-complexing terpyridine ligands form hexagons that assemble into a larger grid by complexing iron(II). The team aims to use these and related supramolecules as single-molecule information storage devices.
Porous ionic liquid captures alcohols and CFCs

This new porous ionic liquid has cavities that are approximately 6.2 Å wide—big enough to capture alcohols and chlorofluorocarbons (CFCs). Previous porous liquids couldn’t grab anything larger than methane or carbon dioxide. Researchers at the University of Cambridge made the new liquid from tetrahedrons of zinc surrounded by coordinating ligands (Nat. Chem. 2020, DOI: 10.1038/s41557-020-0419-2).
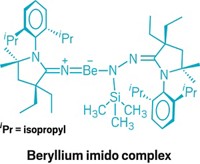
First beryllium radical cation isolated

Chemists at the University of Virginia synthesized this radical that contains beryllium in the +1 oxidation state by oxidizing a previously reported compound containing Be(II) (J. Am. Chem. Soc. 2020, DOI: 10.1021/jacs.9b13777). Compounds with main-group elements with low oxidation states could participate in chemistry typically seen only in transition metals.
Threading multiple rings on a polymer chain
Chemists at Northwestern University designed a molecular machine that uses redox chemistry to thread rings two at a time onto a polymer chain (Science 2020, DOI: 10.1126/science.abb3962). The researchers would like to use this system to build polyrotaxanes for information storage by encoding data in the sequence of rings.
Boron makes quadruple bond


For more than a decade, chemists have thought that rhodium boride contains a triple bond. Using vibrational spectroscopy and theoretical calculations, researchers at Brown University discovered that the bond is likely a quadruple bond instead (J. Phys. Chem. Lett. 2020, DOI: 10.1021/acs.jpclett.9b03484).
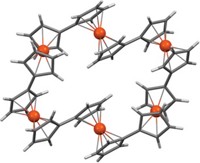
Tying molecular knots
Researchers at the University of Manchester used metal ions to tie molecular strings into two types of knots. Adding lutetium ions alone or in combination with copper ions leads to symmetrical trefoil knots or asymmetrical three-twist knots, respectively (Nature 2020, DOI: 10.1038/s41586-020-2614-0).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter