Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catching the elusive chiral nitrogen compound
Researchers selectively make ammonium cations by trapping the lone electron pair
by Leigh Krietsch Boerner
September 7, 2021

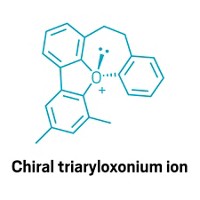
The world of chirality, or the handed-ness of a molecule, is dominated by controlling how atoms and functional groups are arranged around a central carbon. Until now, there hasn’t been a straightforward way for chemists to make compounds with chiral nitrogen centers, important in drugs and biologically active molecules. Matthew Kitching and colleagues at Durham University have found that adding a carbon fragment to an amine and binding the resulting ammonium to a common chiral compound gives chiral N compounds in high specificity, in a single-pot reaction (Nature, 2021, DOI: 10.1038/s41586-021-03735-5).
“Most people don’t think about an amine being chiral because it rapidly interconverts,” Kitching says. The lone electron pair that gives amines their typical trigonal pyramidal shape can tunnel through the N center, flipping the molecule inside out like an umbrella in a windstorm.
By treating an amine with an alkyl bromide compound, Kitching’s team replaces the lone pair with a C substituent, locking the molecules into either the S or the R configuration. Then they combine the resulting enantiomeric mixture of ammonium bromide salts with the chiral compound 1,1’-bi-2-napthol (BINOL). Depending on which isomer of BINOL the team puts in, the compound will make hydrogen bonds preferentially with one enantiomer of the ammonium and form a solid. After recovering the solid by filtration, the team treats it with ethanol and water to remove BINOL, leaving mostly one isomer of the target chiral ammonium compound (shown). Depending on the starting compound, Kitching’s team was able to select which isomer they wanted to make in ratios up to 97:3.
“Wherever we find chirality in chemistry, we know that it has important consequences,” Kitching says. But chemists don’t know what these consequences are for N compounds, because no one’s had a defined path to making them, he says. Now scientists can make enough material and begin to investigate how chiral N centers behave, Kitching says.
Because so few of these compounds exist, spectroscopically identifying the team’s new compounds was a problem. “With nothing to compare to, it’s quite hard to say, definitely, whether you’ve got the R or the S isomer,” Kitching says. They knew they had more of one enantiomer than the other, but they didn’t know which until they ultimately crystallized each of their 32 compounds.
Using the fact that ammonium cations distribute positive charge to their protons to engineer the hydrogen bonding is brilliant, says Cathleen Crudden, an organic chemist specializing in chiral compounds at Queen’s University in Ontario. In addition, performing the entire reaction in just one pot simplifies the synthesis of these chiral ammonium salts, opening up new possibilities for the field, she says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter