Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Electrocatalysis reaction shuttles halides from molecule to molecule
Method could be used to recycle waste dihalides and remediate soil contaminated with organohalogen compounds
by Bethany Halford
January 30, 2021
| A version of this story appeared in
Volume 99, Issue 4

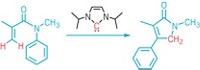
Vicinal dihalides—molecules that feature halogen atoms on adjacent carbons—are useful as flame retardants, as pesticides, and as intermediates en route to polymers and pharmaceuticals. But making these molecules typically requires hazardous chlorine or bromine. Now, chemists led by Bill Morandi at the Swiss Federal Institute of Technology (ETH), Zurich, and Siegfried R. Waldvogel at Johannes Gutenberg University report an electrocatalytic method for making vicinal dihalides from other vicinal dihalides (Science 2021, DOI: 10.1126/science.abf2974). The method zaps simple vicinal dihalides, including 1,2-dibromoethane, 1,1,1,2-tetrachloroethane, and 1,2-dichloroethane, between two graphite electrodes in the presence of an alkene, shuttling two halides from the simple molecule to the more complex one (example shown). Because the reaction also works in reverse, chemists could use it to recycle vicinal dihalide waste. For example, Morandi, Waldvogel, and coworkers take soil contaminated with the persistent pollutant lindane, a cyclohexane molecule with a chlorine on each carbon that was once used as a pesticide, and electrocatalytically transform it into benzene and simple vicinal dihalides. They perform the transformation directly on contaminated soil, showing that the shuttling reaction tolerates biological and mineral impurities that might be present in the soil.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter