Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Making new molecules by adding carbons one at a time
Method allows for more select editing of complex molecules
by Leigh Krietsch Boerner
February 14, 2023

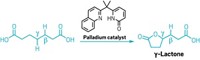
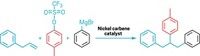
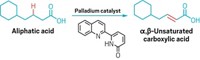
Chemists have increased their power to make a variety of compounds by figuring out how to add a single carbon to simple amides, creating four new C bonds in a single step. Mamoru Tobisu and coworkers at Osaka University used an N-heterocyclic carbene, a stable compound with two unpaired electrons at the C, to make a variety of cyclic amides called lactams with a variety of side groups (Science 2023, DOI: 10.1126/science.ade5110).
In the past, single carbon atoms have been too unstable to be useful in organic synthesis. In this method, the researchers gamed the system by using the carbene as a stable precursor to an atomic carbon, creating a more practical way to add just one C atom at a time. The group was initially working on a reaction that used the carbene as a catalyst but were surprised to find products that had one extra C, Tobisu says in an email.
One of the main goals of organic synthesis is to create complex molecules that can be useful—as drugs or in new materials, for example. Chemists need to do this as efficiently as possible, Tobisu says. This ability to add a single carbon in one step could potentially shorten synthetic processes to create more elaborate compounds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter