Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Molecular-editing reaction expands indoles with N
New method adds another nitrogen into bioactive motif commonly found in drugs
by Bethany Halford
September 8, 2022
| A version of this story appeared in
Volume 100, Issue 32
Indoles abound in bioactive molecules. They make up the molecular core of the essential amino acid tryptophan, the sleep-inducing supplement melatonin, and the psychedelic compound psilocybin. Chemists have now devised a way to plug a second nitrogen into indole’s five-membered ring to generate either quinazolines or quinoxalines, depending on the indole’s substitution pattern. Because the transformation tolerates a wide range of functional groups, it can help make new molecules from indoles during the late stages of a chemical synthesis—a boon to medicinal chemists looking to tweak pharmaceutical leads.

Chemists in Bill Morandi’s lab at the Swiss Federal Institute of Technology (ETH), Zurich, developed the new reaction. Julia C. Reisenbauer, the graduate student who initiated the project, says she was inspired by the Ciamician-Dennstedt rearrangement—a reaction first reported in 1881 that adds a carbon atom into indoles and pyrroles. To create a version of this transformation that adds a nitrogen instead of a carbon, the chemists hit upon using an iodonitrene reagent they generate in the reaction flask from a hypervalent iodine compound known as PIFA and ammonium carbamate (Science 2022, DOI: 10.1126/science.add1383).
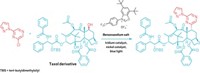
Reisenbauer says she originally explored metal catalysis, an area that Morandi’s lab specializes in, to help squeeze a nitrogen into indole rings. “But in the end, it turned out that the key was to use iodonitrene reagents,” she says. The researchers also found they needed to append a silyl protecting group to the indole’s nitrogen. This group prevents unwanted side reactions and steers the reaction to the desired quinazoline or quinoxaline product. The chemists used the reaction on various indoles. Starting with pindolol, a drug used to treat high blood pressure, they created a quinazoline derivative. They also used tryptoline-3-carboxylic acid, analogs of which have been studied as antidiabetic agents, to make a quinoxaline derivative (both reactions shown).
Richmond Sarpong, a synthetic organic chemist at the University of California, Berkeley, who specializes in developing molecular-editing reactions, says inserting a nitrogen into heterocycles, as Morandi and his coworkers have done, is a long-sought goal. “The functional group compatibility is particularly impressive in this method,” he says in an email. “This is a powerful demonstration of late-stage modification of molecules at their core, which will add another method for diversification to the toolbox of the medicinal chemist.”
“Taking the mechanistic template of the Ciamician-Dennstedt rearrangement and using it to insert nitrogen instead of carbon was insightful,” Mark D. Levin says in an email. Levin’s group at the University of Chicago reported a reaction last year that snips nitrogen atoms out of molecules. “I think the most striking thing about this paper is what it says about the popularity of single-atom skeletal editing when you have people like Morandi, who had previously been focused elsewhere, starting to invest time and effort into the problem.”
CORRECTION:
This story was updated on Nov. 2, 2022 to correct the structure of PIFA. The incorrect structure was missing two oxygen atoms.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter