Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
New mapping method reveals reactions galore
System combines compounds in unfamiliar ways
by Leigh Krietsch Boerner
April 1, 2020
| A version of this story appeared in
Volume 98, Issue 13

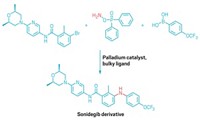
It’s dinnertime, and the only food in your house is a can of beans and a box of spaghetti. What can you make? A new mapping method that combines known reactants in unfamiliar ways can help organic chemists solve a similar problem. Instead of reacting an amine and a carboxylic acid in the usual amide-coupling reaction, Tim Cernak and coworkers from The University of Michigan figured out that chemists can combine these compounds in 320 different ways (Nature 2020, DOI: 10.1038/s41586-020-2142-y).
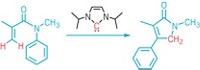
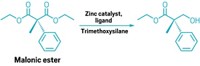
Cernak and coworkers created a mathematical model by taking all the possible ways an amine and a carboxylic acid can combine, and using this equation to find a number of potential products. For example, the two starting materials can combine to form the amide, but they can also go through a decarboxylation to form an amine, or a tandem decarboxylation–deamination to give a new C–C bond (shown). Using their system, the team found that amines and carboxylic acids can combine in 80 coupling patterns. When they considered all the combinations of sp2 or sp3 hybridization, this led to 320 potential products.
“Our only filter was the octet rule,” he says. “If you can draw it on the chalkboard, and the product observes the octet rule, there must be some way to make it.”
Of the 320 predicted reactions, the team tried 15 in the lab, some of which were already known and some of which were new. They all worked, giving different products depending on the reaction conditions, from 45–91% yields. In addition, the team used the 320 potential products they found as substructures to search 9,279 pharmaceuticals and natural products from the DrugBank database, and found thousands of hits. This means that the two starting materials, an amine and a carboxylic acid, can be combined in a myriad of different ways to form molecules of interest, such as pharmaceuticals and natural products. Many potential reactions that the team found don’t yet exist, Cernak says. “We’re going to try and make them exist and use high throughput tools to do that.”
This way of approaching synthesis kind of turns current thinking on its side. Instead of using the starting materials to get a molecule with specific properties, Cernak wants to be able to use the reaction itself. So instead of changing the starting materials, a scientist could use the same starting materials under different reaction conditions, such as a different catalyst, reagents, and reaction conditions to get a different result.
It’s very creative to make diverse products with diverse transformations and conditions, says Connor Coley, an organic chemist from the Massachusetts Institute of Technology. “There may be far more ways to combine pairs of in-stock building blocks than meets the eye.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter