Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Perfluorinated ligand makes long-awaited debut in rhodium complex
After a 40-year wait, fully fluorinated cyclopentadienyl derivative finally connects with a metal partner
by Mark Peplow, special to C&EN
August 30, 2022

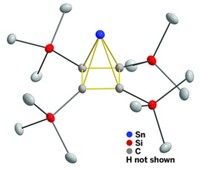
The first coordination complex to contain a [C5(CF3)5]− ligand has been created, more than 40 years after the ligand itself was first synthesized (Angew. Chem. Int. Ed. 2022, DOI: 10.1002/anie.202211147).
The ligand is a descendant of cyclopentadienyl (Cp), found in the iconic sandwich complex, ferrocene. One common variant of this ligand is known as Cp*, in which each of the five cyclopentadienyl carbons carries a methyl group. The new ligand is the perfluorinated version of Cp*, in which every hydrogen has been replaced by fluorine.
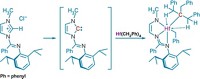
All previous attempts to coordinate the ligand to a metal had failed because its CF3 groups are so strongly electron withdrawing. “There’s almost no electron density in the aromatic system, so it wasn’t expected to be a coordinating ligand,” says graduate student Robin Sievers, who led the experimental work in Moritz Malischewski’s group at the Free University of Berlin.
The team overcame this hurdle by combining the ligand with an electron-rich rhodium cyclooctadiene (COD) precursor. Rhodium contributes bonding electrons back to the ligand to form the weakly bound complex Rh(COD)(C5(CF3)5), a yellow solid that is stable at room temperature.
The perfluorinated Cp* is easily displaced by other ligands, so the researchers hope the complex might be exploited to make new catalysts—particularly those that could be used in conjunction with fluorinated solvents.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter