Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Reaction uses alternating current to selectively reduce carbonyl groups
A simple and standard electrochemistry setup overcomes a hurdle in the field
by Fernando Gomollón-Bel, special to C&EN
October 25, 2021

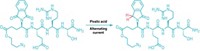
Scientists at Scripps Research have used rapid alternating current (AC) to selectively reduce carbonyl groups (J. Am. Chem. Soc. 2021, DOI: 10.1021/jacs.1c06572). The technique avoids side reactions with other redox-active groups—a characteristic of some reactions that use direct current (DC)—solving a critical challenge in the field of electrosynthesis.
“We always wondered if there would be anything unique in AC electrochemistry, since direct current is pretty much the only way for electrochemical synthesis,” explains Phil S. Baran, who led the Scripps team. Previously, chemists disregarded AC energy sources because they generated complicated mixtures, as well as lots of heat and gas emissions. However, Baran’s team noticed that a type of alternating current called rapidly alternating polarization was remarkably good at reducing phthalimide. In that experiment, direct current yielded a “sluggish reaction,” whereas rapidly alternating polarity gave total reduction of one of phthalimide’s carbonyl groups under otherwise identical conditions. “We decided to deep dive into the unique effect of alternating polarity on chemoselective synthesis,” he says.
The team then tested the approach on more complex molecules. They picked a synthetic tetrapeptide featuring eight redox-active functional groups, among them a phthalimide residue. The reaction reduced the phthalimide with 52% conversion and avoided the other groups.
Song Lin, an expert in electrosynthesis at Cornell University who was not involved in the work, calls it “stunning.” Reducing such a complex molecule selectively is “really difficult using traditional chemical reductants,” he adds. Lin sees “great potential” in this strategy, particularly in fields like medicinal chemistry.
Unlike other reduction reactions, this electrochemical process works through a single-electron transfer. “It’s an intricate interplay between electron transfer kinetics and reversibility,” Lin says. “The desired reaction is faster, whereas competing reactions are slower, more reversible,” he adds. “This will get people thinking about new creative uses for AC.”
The work was carried out in collaboration with instrument manufacturer Ika. The company developed a free software update to enable new AC functionalities on its commercially available electrosynthesis device ElectraSyn. Baran has collaborated with Ika for several years but receives no commissions from the sale of ElectraSyn equipment; lead author Yu Kawamata serves as a paid consultant for the company.
“This paper takes electrosynthesis a step further,” with a strategy that is readily available to synthetic chemists, targets individual functional groups, and is environmentally friendly, says Shelley D. Minteer, an electrochemist at the University of Utah who was not involved in the work. For molecules this complex, DC electrolysis would be a challenging process, she adds.
Minteer believes this technique could be adapted to many different functional groups just by tuning the electrochemical parameters. Indeed, Baran says “this reactivity is only a snapshot” of the system’s current capabilities. By altering the waveform and potential of the alternating current, the group has achieved “a number of unique reactions,” some of which strongly deviate from traditional organic chemistry expectations, he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter