Physical Chemistry
Massive tools advanced big chemistry in 2022
Gigantic data sets and colossal instruments helped scientists tackle chemistry on a giant scale this year
by Ariana Remmel

Scientists made big discoveries with supersized tools in 2022. Building on the recent trend of chemically competent artificial intelligence, researchers made great strides, teaching computers to predict protein structures on an unprecedented scale. In July, Alphabet-owned company DeepMind published a database containing the structures of almost all known proteins—200 million-plus individual proteins from over 100 million species—as predicted by the machine learning algorithm AlphaFold. Then, in November, the tech company Meta demonstrated its progress in protein prediction technology with an AI algorithm called ESMFold. In a preprint study that has not yet been peer-reviewed, Meta researchers reported that their new algorithm is not as accurate as AlphaFold but is quicker. The increased speed meant that the researchers could predict 600 million structures in just 2 weeks (bioRxiv 2022, DOI: 10.1101/2022.07.20.500902).
Biologists at the University of Washington (UW) School of Medicine are helping expand computers’ biochemical capabilities beyond nature’s template by teaching machines to propose bespoke proteins from scratch. UW’s David Baker and his team created a new AI tool that can design proteins by either iteratively improving on simple prompts or by filling in the gaps between selected parts of an existing structure (Science 2022, DOI: 10.1126/science.abn2100). The team also debuted a new program, ProteinMPNN, that can start from designed 3D shapes and assemblies of multiple protein subunits and then determine the amino acid sequences needed to make them efficiently (Science 2022, DOI: 10.1126/science.add2187; 10.1126/science.add1964). These biochemically savvy algorithms could aid scientists in building blueprints for artificial proteins that could be used in new biomaterials and pharmaceuticals.
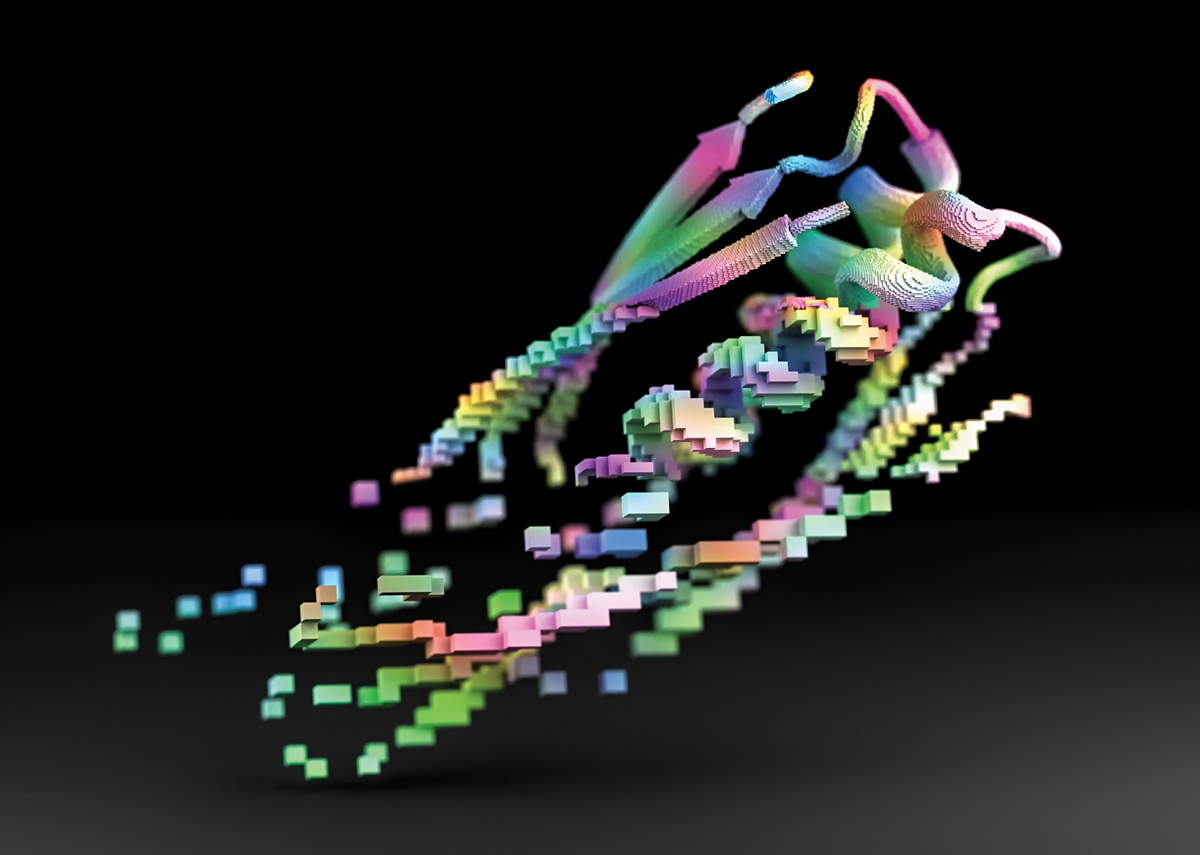
As computational chemists’ ambitions grow, so do the computers used to simulate the molecular world. At Oak Ridge National Laboratory (ORNL), chemists got a first glimpse at one of the most powerful supercomputers ever built. ORNL’s exascale supercomputer, Frontier, is among the first machines to calculate more than 1 quintillion floating operations per second, a unit of computational arithmetic. That computing speed is about three times as fast as the reigning champion, the supercomputer Fugaku in Japan. In the next year, two more national laboratories plan to debut exascale computers in the US. The outsize computer power of these state-of-the-art machines will allow chemists to simulate even bigger molecular systems and on longer timescales. The data collected from those models could help researchers push the boundaries of what’s possible in chemistry by narrowing the gap between the reactions in a flask and the virtual simulations used to model them. “We’re at a point where we can start really asking questions about what is it that’s missing from our theoretical methods or models that would get us closer to what an experiment is telling us is real,” Theresa Windus, a computational chemist at Iowa State University and project lead with the Exascale Computing Project, told C&EN in September. Simulations run on exascale computers could help chemists invent novel fuel sources and design new climate-resilient materials.
Across the country, in Menlo Park, California, the SLAC National Accelerator Laboratory is installing supercool upgrades to the Linac Coherent Light Source (LCLS) that could allow chemists to peer deeper into the ultrafast world of atoms and electrons. The facility is built on a 3 km linear accelerator, parts of which are cooled with liquid helium down to 2 K, to produce a type of superbright, superfast light source called an X-ray free-electron laser (XFEL). Chemists have used the instruments’ powerful pulses to make molecular movies that have enabled them to watch myriad processes, such as chemical bonds forming and photosynthetic enzymes going to work. “In a femtosecond flash, you can see atoms stand still, single atomic bonds breaking,” Leora Dresselhaus-Marais, a materials scientist with joint appointments at Stanford University and SLAC, told C&EN in July. The upgrades to LCLS will also allow scientists to better tune the energies of X-rays when the new capabilities become available early next year.

This year, scientists also saw just how powerful the long-awaited James Webb Space Telescope (JWST) could be for revealing the chemical complexity of our universe. NASA and its partners—the European Space Agency, the Canadian Space Agency, and the Space Telescope Science Institute—have already released dozens of images, from dazzling portraits of stellar nebulae to the elemental fingerprints of ancient galaxies. The $10 billion infrared telescope is decked out with suites of scientific instruments designed to explore the deep history of our universe. Decades in the making, the JWST has already outperformed the expectations of its engineers by snapping an image of a whirling galaxy as it appeared 4.6 billion years ago, complete with spectroscopic signatures of oxygen, neon, and other atoms. Scientists also measured signatures of steamy clouds and haze on an exoplanet, providing data that could help astrobiologists search for potentially habitable worlds beyond Earth.




![A molecular model shows how the largest of the new cavitands binds C<sub>70</sub>. A structure of acridane[4]arene A molecular model shows how the largest of the new cavitands binds C<sub>70</sub>. A structure of acridane[4]arene](/content/dam/cen/100/44/WEB/10044-cover5-cavitand-combined.jpg)













Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter