Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biocatalysis
Enzyme doesn’t need oxygen to make ethylene
Finding offers a possibly safer biosynthetic route to the important feedstock
by Leigh Krietsch Boerner
September 4, 2020
| A version of this story appeared in
Volume 98, Issue 34

The chemical industry makes about 25 million metric tons of ethylene a year and uses the compound to manufacture plastics, polymers, and longer-chain carbon compounds. Scientists want to engineer bacteria to make this vital chemical feedstock to avoid using fossil fuels. But so far they can’t do so on a large enough scale to satisfy industry’s needs. Also the enzymes currently used by engineered bacteria to make ethylene need oxygen to function, which creates a safety issue, because O2 plus ethylene is an explosion risk.
A group from the Ohio State University, Oak Ridge National Laboratory, Pacific Northwest National Laboratory, and Colorado State University has discovered a bacterial enzyme that can produce ethylene and methane without needing O2 (Science 2020, DOI: 10.1126/science.abb6310). This ethylene-making enzyme could potentially offer a safer path toward synthesizing the valuable chemical.
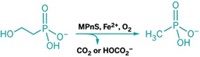
The bacteria come from both soil and fresh water. Basically, these microbes take bits of leftover metabolic waste that contain sulfur and break down those molecules to 2-(methylthio)ethanol, says Justin North, a research microbiologist at Ohio State and the first author on the paper. Then, the methylthio-alkane reductase takes the sulfur from that molecule and releases ethylene (shown). The enzyme can produce methane from dimethyl sulfide in the environment, he says. The bacteria only use these reactions in low-sulfur conditions as a way to scavenge for the element.
Cells need sulfur to make important biomolecules such as methionine and adenosyl-methionine. Scientists didn’t know where bacteria got the sulfur to make these compounds in low-oxygen conditions, because in such environments there isn’t much sulfate, the bacteria’s preferred sulfur source.
The ethylene-O2 safety issue is a large concern for finding a biosynthetic route to the olefin, says Jianping Yu, a molecular biologist at the National Renewable Energy Laboratory in Golden, Colorado. “The discovery of an enzyme/pathway for ethylene synthesis under anaerobic conditions bypasses this safety risk, and could open up a new research direction for biological ethylene production.”
North says that the group is now working on engineering the bacteria to make more ethylene.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter