Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Boronate rearrangement gets an enantioselective makeover
Chemical sleuthing transforms the decades-old Matteson reaction into a tool for building stereocenters
by Bethany Halford
November 4, 2021
| A version of this story appeared in
Volume 99, Issue 41

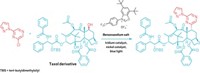
The way an atom’s substituents are arranged around a stereocenter can mean the difference between a potent drug and a compound that’s harmful. So chemists are always on the lookout for transformations that build stereocenters selectively—so called enantioselective reactions. A team of researchers now reports an enantioselective, catalytic version of the Matteson reaction. The new take generates a chiral carbon attached to two reactive handles—a chlorine and a boronic ester. This intermediate can then be converted into myriad trisubstituted stereocenters using other stereospecific reactions.
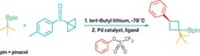
The Matteson reaction—first reported by Washington State University’s Donald S. Matteson in the 1960s—involves the displacement of a leaving group, such as a halide, from the α-carbon of an alkyl boronic ester to create a new bond. “It’s a well-known reaction, but nobody’s ever been able to do it catalytically and with enantioselectivity,” says Harvard University’s Eric N. Jacobsen, who developed the new reaction with graduate students Hayden A. Sharma and Jake Z. Essman.
Chemists in Jacobsen’s group have been using chiral thiourea catalysts for enantioselective reactions, and they wondered if their system was compatible with the Matteson reaction. They had doubts because their catalysts work by making hydrogen bonds, and the Matteson reaction uses organometallic reagents that are strong bases, which aren’t compatible with hydrogen-bond donors.
“What we discovered ended up being much more interesting than we could have hoped,” Jacobsen says. Surprisingly, the reaction worked best if the researchers mixed their catalyst with a very strong base. After some chemical detective work, they found that the catalyst they were generating was quite different from the starting hydrogen-bonding catalyst. In fact, the catalyst loses all its acidic protons to form a lithium-isothiourea-boronate complex (Science 2021, DOI: 10.1126/science.abm0386).
The chemists make a dichlorinated boronate by first mixing a boronic ester with dichloromethane and lithium diisoproplylamide. The catalyst complex then enantioselectively plucks off one of the chlorides from the boronate. The resulting intermediate rearranges to form an α-chloro pinacol boronic ester. From there, Jacobsen says, “there’s an enormous range of products that we can make.”
“I am very happy to see this development,” says Matteson in an email. “I have long been a bit frustrated that I couldn’t figure out a way to catalyze that reaction with a chiral catalyst. Now it can become much more useful in synthesis.”
The catalyst is not currently commercially available, and Jacobsen says that its synthesis requires several steps. Moving forward, he says, his group is hoping to simplify the catalyst to make its synthesis more practical. Jacobsen also says that his group is exploring other reactions with the lithium-isothiourea-boronate complexes. “There’s a lot of chemistry that you can imagine doing with these types of catalysts,” he says.
Varinder Aggarwal, an organic chemist at the University of Bristol, who also works on the Matteson reaction, calls the transformation “an amazing piece of work.” He says in an email that “the discovery is remarkable in that it is hard to imagine a catalyst that is capable of performing such a role, and even more remarkable when it is found that the catalyst works in a much more complex manner and one that really could not have been planned.” The system, Aggarwal says, “opens up a new mode of activation and it will be interesting to see where else this might be applied.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter