Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Catalyst chirality switched with a flash of light
Molecular motor and chiral ligand combined to create photoresponsive catalyst
by Laura Howes
December 4, 2018

Cells have a lot of control over enzymes. They can modify an enzyme to be more or less active, or to change the reaction that it catalyzes. But chemistry in the lab is different. “Normally when we design a ligand or catalyst we design it to do one specific transformation,” explains Ben Feringa of the University of Groningen, who won the 2016 Nobel Prize in Chemistry. “What we wanted to do is make an adaptive catalyst.”
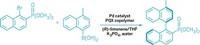
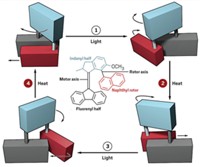
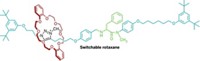
To do that, Feringa’s group has combined a molecular motor with an existing asymmetric catalyst. The result is a catalyst that can switch its chirality, and as a result the stereochemistry of its products, with a flash of light (J. Am. Chem. Soc.2018, DOI: 10.1021/jacs.8b10816).
The switchable catalyst features what is known as axial chirality. This type of chirality is often used in asymmetric catalysis and results from the nonplanar arrangement of groups around an axis. Feringa’s molecule contains a biphenol group with axial chirality and a rigid core that rotates when hit with ultraviolet light. Because of the tight conformational coupling of these two elements, switching the helicity around the axis also changes the chirality of the biphenol. The biphenol coordinates with a zinc ion to form the active catalyst.
As a proof of principle, the chemists tested the catalyst in an organometallic 1,2-addition reaction to produce different stereoisomers depending on which form of the catalyst was used.
The catalyst is “a real achievement in the dynamic coupling of several types of convoluted chiralities,” says Nicolas Giuseppone, an organic chemist at the University of Strasbourg.
“It nicely demonstrates that switchable chiral catalysts can be obtained by the designed integration of functional units with well-defined stereochemical properties,” adds Alberto Credi of the University of Bologna. “Such a modular approach could in principle be applied to a wealth of biaryl structures,” he adds, suggesting this could lead to various chiral switches that could control different processes.
Feringa agrees. The dream, he says, is adaptive systems that could be switched from one reaction to another using an external signal. But, he admits, “I don’t know where the most useful applications will be.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter