Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Chemistry In Pictures
Chemistry in Pictures: Bubbling catalyst
by Alexandra Taylor
August 1, 2019

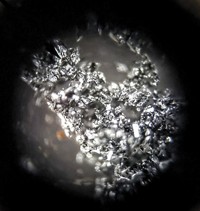
These photos show a boron-dipyrromethene (BODIPY) derivative under white light (left) and ultraviolet light. Chris Thomson, a PhD candidate working in Filipe Vilela’s lab at Heriot-Watt University, took the photos after purifying the BODIPY-based photocatalyst. After most of the solvent had evaporated off, Thomson tried to get the concentrated, oily residue to solidify by reducing the pressure. The remaining solvent became trapped in bubbles in the viscous residue. As the bubbles expanded, they pushed the concentration of the product—which was still slightly dissolved—past a critical point, and it rapidly solidified as a thin, crystalline film around the bubbles. Thomson says the vaporized solvent escaped through little holes, but the film remained stable. “The product has many potential applications, but in the immediate future I’m intending to use it to generate a polymer-supported organic photocatalyst for generating reactive oxygen species, useful for organic synthesis,” Thomson explains. The Vilela lab is researching ways to make heterogeneous photocatalysts more efficient.
Submitted by Chris Thomson/@VilelaLAB
Do science. Take pictures. Win money. Enter our photo contest here.
Related C&EN content:




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter