Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Customizing alloy catalysts for hydrogenation
Study shows how to controllably boost activity by fine-tuning catalyst composition
by Mitch Jacoby
February 27, 2022
| A version of this story appeared in
Volume 100, Issue 8
When it comes to catalytic activity, more isn’t always better. Overly active catalysts may be hard to control; finding the sweet spot can be tricky. That job may be easier now thanks to a study showing the effects of fine-tuning the composition of catalytic alloys (Nat. Chem. 2022, DOI: 10.1038/s41557-021-00855-3).
Manufacturers make tens of millions of metric tons of polyethylene annually. The ethylene starting material typically contains trace amounts of acetylene, which must be removed because it can poison polymerization catalysts. Polymer suppliers generally remove the impurity using palladium catalysts to convert acetylene to ethylene. But palladium’s high activity means the catalyst can overhydrogenate acetylene, forming unwanted ethane and generating dangerous levels of heat.
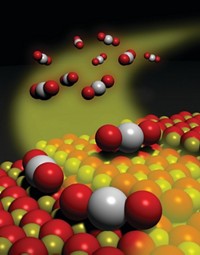
To help tackle the problem, Michael J. Janik, Robert M. Rioux, and coworkers at Pennsylvania State University studied a family of palladium-zinc alloys. They used solid-state synthesis methods to precisely tailor the alloys, forming zinc-based metals with various types of catalytically active sites—isolated palladium atoms, palladium trimers, and trimers in which two palladium atoms were bridged by a third metal.
Catalysis tests and quantum calculations showed that small changes in the Pd–Zn ratio led to enormous differences—four orders of magnitude—in hydrogenation activity driven by the presence of overly active palladium trimers in some of the alloys. The study also showed that the high activity could be maintained but controlled by breaking up palladium trimers with an atom of copper or gold, providing clues for designing industrial catalysts.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter