Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Eliminating the middleman improves production of clean-burning hydrogen fuel
Electronically linking the catalyst to the electrode simplifies electrochemical reactions
by Mitch Jacoby
August 27, 2019
| A version of this story appeared in
Volume 97, Issue 34
By electronically linking a catalytic molecule to a graphite electrode, researchers have come up with a general way to make better catalysts for electrochemical reactions. The advance, presented at the American Chemical Society national meeting in San Diego on Monday, may lead to new strategies for designing catalysts that mediate water splitting to generate clean-burning hydrogen fuel.
Electrocatalysts are key to carrying out reactions such as water splitting speedily and efficiently. With the right catalyst, researchers can use electrical energy to split water into oxygen gas and clean-burning hydrogen fuel. The mediator serves as a molecular middle man, passing electrical energy from the electrode to the reactant molecule and steering the reactant’s transformation through a series of steps.
For years, researchers have focused on finding just the right mediator for water splitting and other electrochemical reactions. Several highly active ones have been found, but there are tradeoffs. To do its job, the mediator first undergoes a redox change then drives the catalytic reaction. That stepwise arrangement impedes the catalytic reaction because the mediator’s “needs” must be met. For example, the reaction must be run at voltages and currents that suit the mediator—not just the reactant. And the reaction won’t proceed until the mediator forms a high-energy redox intermediate.
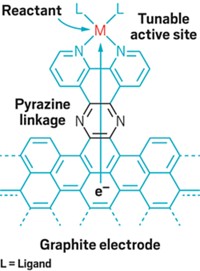
Yogesh Surendranath of the Massachusetts Institute of Technology reasoned that chemically binding a mediator to a sheet of conjugated six-membered rings in a graphite electrode—in effect making the electrode and mediator a single conjugated molecular entity—would bypass the middleman arrangement and the limitations that go with it.
At the meeting, Surendranath reported that members of his group including Megan N. Jackson had made this strategy work. They synthetically conjugated a water-splitting catalyst, an organorhodium complex, to a graphite electrode. This complex drives the hydrogen evolution reaction (HER), which combines protons and electrons to form molecular hydrogen.
Speaking at a symposium organized by the Division of Inorganic Chemistry, Surendranath described his group’s investigation. They carried out control tests comparing the rhodium complex in graphite-conjugated and unconjugated forms. The group’s spectroscopy results indicate that whereas in the controls, the reaction occurs through a stepwise pathway involving redox intermediates, the conjugated catalyst instead facilitates a direct route (J. Amer. Chem. Soc. 2019, DOI: 10.1021/jacs.9b04981).
Describing the conjugation strategy as “creative,” Jillian L. Dempsey of the University of North Carolina at Chapel Hill remarked that by side-stepping the complex redox pathways, the researchers have found a new approach to HER. She added that she expects this approach may have applications for making other, more efficient fuel-forming catalysts.
The team provided a top-to-bottom demonstration of the unique way in which a molecular electrocatalyst functions when it is tied via conjugation to the electronic band structure of the electrode, said Jeff Warren, a catalysis specialist at Simon Fraser University. ““This work is an important advance with broad implications for catalyst design,” he said.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter