Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Enzyme smooths path to planar chiral macrocycles
Good yields and enantioselectivity abide
by Leigh Krietsch Boerner
February 23, 2020
| A version of this story appeared in
Volume 98, Issue 8
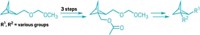
Planar chiral molecules are relatively common in natural products, kind of funny looking, and largely ignored. Though they can be useful as pharmaceuticals or agrochemicals, they are difficult to make. Now, Shawn Collins and coworkers at the University of Montreal have developed an enzyme-catalyzed synthesis to form planar chiral molecules with high enantioselectivity (example shown) from readily available starting materials, which can be easily functionalized (Science 2020, DOI: 10.1126/science.aaz7381). The team made target compounds with up to 99% yield and 66–99% enantioselectivity. Instead of a more traditional metal catalyst, which can create toxic waste, Collins decided to look for one with a biological origin. The serine hydrolase Candida antarctica lipase B “is the organic chemist’s dream because it’s an enzyme that is superstable to high temperatures and actually more active in organic solvents than in water,” he says. The natural enzymatic pocket evolved to accommodate a secondary alcohol, so Collins was surprised that the team’s large molecules fit. But it can fit only so much, so the group couldn’t make planar chiral macrocycles with larger side groups like styrenes. But they can include halogens, he says, which are a great handle for functionalities.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter