Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Ir catalyst can functionalize remote C–H bonds
Chiral cave modifies fatty acid derivatives
by Leigh Krietsch Boerner
August 20, 2020
| A version of this story appeared in
Volume 98, Issue 32
RETRACTION
The paper described in this article was retracted on April 28, 2022 (Science 2022 DOI: 10.1126/science.abq4856).
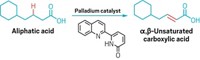
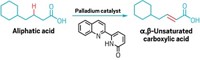
At the moment, pharmaceuticals are far from green—the carbon atoms that make up their backbones come from oil. As an alternative, scientists can get carbon chains via fatty acids from biomass. However, fatty acid derivatives contain carboxyl groups, which make it difficult to functionalize distant carbons. Researchers now have developed a catalyst that can do much more with these green starting materials.
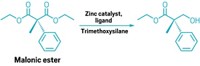
Masaya Sawamura and coworkers from Hokkaido University designed an iridium catalyst with a “chiral cave” that locks onto substrates and borylates them at a particular spot, three carbons away from the carboxyl group. The catalyst reacts with a variety of fatty acid derivatives to make 30 compounds at an enantiomeric purity of greater than 90% and good yields (Science 2020, DOI: 10.1126/science.abc8320).
Through a series of calculations, Sawamura and coworkers found that the catalyst assembles to form a pocket, akin to an enzyme active site, that binds the compound through multiple noncovalent interactions. These include π-π interactions between a monophosphite ligand and a urea-pyridine receptor ligand, coordination of the pyridine to the Ir center, and hydrogen bonding between the substrate and the urea ligand. The modularity of the catalyst is “reminiscent of a machine built by piecing together individual parts,” Sawamura says. It should also enable flexibility in changing the parts of the catalyst to potentially expand reactivity.
Starting with aliphatic secondary and tertiary amides and esters, the researchers made a series of borylated compounds. These could then be transformed into pharmacologically important compounds, including a derivative of the neurotransmitter GABA.
Jin-Quan Yu, an organic chemist at Scripps Research Institute, calls the work “another great example of controlling site selectivity in C–H activation by remote directing.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter