Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Iron catalyst converts CO₂ to jet fuel
Inexpensive material and procedure turn troublesome greenhouse gas into valuable product
by Mitch Jacoby
January 8, 2021
| A version of this story appeared in
Volume 99, Issue 2

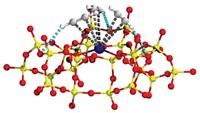
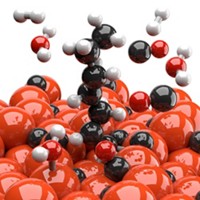
Peter P. Edwards predicts that future generations will look back at the way today’s scientists grappled with greenhouse gas problems and ask incredulously, “Bury CO2 in the ground? Was that the best you could come up with?” His group at the University of Oxford thinks that converting the troublemaker gas to valuable compounds is a better way to go. To that end, Edwards, Benzhen Yao, Tiancun Xiao, and coworkers have come up with an inexpensive iron catalyst that converts carbon dioxide to jet fuel, a mixture of hydrocarbons in the C8–C16 range (Nat. Commun. 2020, DOI: 10.1038/s41467-020-20214-z). The team prepared a number of catalysts using a one-pot aqueous method in which they mixed iron nitrate and other starting materials with citric acid or other cation-complexing agents. Then they burned the resulting pastes in air, forming nanostructured catalysts, and used them to hydrogenate CO2 to make jet fuel. Tests comparing the effects of adding various transition metals and alkali metals showed that doping the Fe-based compound with manganese and potassium improved its catalytic performance. Results show that under mild conditions, the unoptimized Fe-Mn-K catalyst converts roughly 40% of CO2, producing C8–C16 hydrocarbons with nearly 50% selectivity and a mixture of light olefins, which are also industrially valuable.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter